ELECTRONEGATIVITY
Electronegativity (EN) of an atom in a molecule is defined as its tendency to attract the bonding electrons in a chemical bond towards itself.
In a heteronuclear chemical bond X―Y between two different atoms X and Y, [where the electronegativity of X and Y are different] the bonding electron pair will be shifted more towards the atom of greater electronegativity.
The electronegativity value of an atom is not a fixed quantity. Electronegativity value of an atom depends on the bonding situation. Different electronegativity values of an atom may be assigned in different bonding environment. So, electronegativity is not an inherent property of an atom.
Scales of Electronegativity
Different chemists have been defined the electronegativity term in different ways. Different chemists have proposed different scales of electronegativity.
Pauling’s Scale of Electronegativity
According to Pauling, electronegativity of an atom in a molecule is its power to attract the bonding electrons towards itself. A―A and B―B are two covalent molecules, react to form A―B covalent molecule---
A―A + B―B -----> A―B + A―B
According to Pauling, the bond dissociation energy of A―B covalent bond (EA―B) is greater than the geometric mean of the bond dissociation energies of A―A (EA―A) and B―B (EB―B) covalent bonds, therefore---
EA―B > √ (EA―A x EB―B)
The difference between EA―B and [√(EA―A x EB―B)] is called ionic resonance energy of A―B bond. This ionic resonance energy is denoted by ΔA―B. Therefore---
ΔA―B = EA―B – √(EA―A x EB―B)
Or, √(ΔA―B) = √[EA―B – √(EA―A x EB―B)] ----- (1)
Where, √ΔA―B is a measure of ionic character in A―B covalent bond. The amount of ionic character in A―B covalent bond increases with the increase in the magnitude of √ΔA―B. Therefore---
√(ΔA―B) ∝
Suppose, XA is the electronegativity of A atom and XB is the electronegativity of B atom. If the electronegativity of A atom is greater than the electronegativity of B atom, therefore, (XA – XB) value increases with the increases in the amount of ionic character in A―B bond. So--
(XA – XB) ∝
From equation (2) and (3), we get---
(XA – XB) ∝
Or, (XA – XB) = K √(ΔA―B)
Where K is a constant.
Putting the value of [√(ΔA―B)] from equation 1, we get---
(XA – XB) = K √[EA―B – √(EA―A x EB―B)] ----- (4)
When bond dissociation energies are expressed in Kcal/mol, the equation (4) becomes---
(XA – XB) = 0.208 √[EA―B – √(EA―A x EB―B)] ----- (5)
When bond dissociation energies are expressed in KJ/mol, the equation (4) becomes---
(XA – XB) = 0.102 √[EA―B – √(EA―A x EB―B)] ----- (6)
Equation (5) and (6) are called the Pauling’s equation.
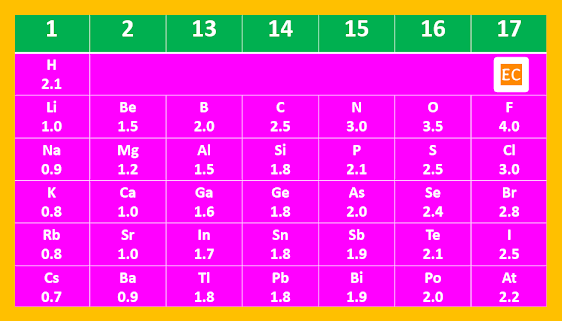
Pauling arbitrarily assumed the electronegativity of hydrogen, that is XH is equal to 2.1 and calculate the electronegativity value of other atom with the help of these equation and framed his electronegativity scale---
Mulliken’s Approach
Mulliken proposed that the average of ionisation energy (IE) and electron affinity (EA) is proportional to the electronegativity (EN) of an element. Therefore---
EN ∝
He proposed that when both IE and EA are in electron volt unit, the electronegativity can be calculated with the help of the following equation---
EN = 0.374 [(IE + EA) / 2] + 0.17
Or, EN = 0.187 [(IE + EA)] + 0.17
Though Mulliken’s approach is far-reaching, the main disadvantage is that, electron affinity values are not known except in a few cases.
Allred and Rochow’s Scale of Electronegativity
According to Allred and Rochow the electronegativity of an atom can be calculated by the following formula---
EN = [(0.359 x Zeff) / r2] + 0.744
Or, EN = [{0.359 x (Z – σ)} / r2] + 0.744
Where---
Zeff = Effective nuclear charge
Z = Nuclear charge
σ = Screening or shielding constant
r = Covalent radius in A0 unit
Periodic Variation of Electronegativity
Electronegativity increases on moving from left to right along a period with increase in nuclear charge. With increase in nuclear charge the radius of atom decreases, therefore electronegativity value increases.
Electronegativity decreases on moving from top to bottom along a group with increase in atomic radius.
Factors Affecting the Magnitude of Electronegativity
(1) Size of the atom---
Electronegativity ∝
The tendency of smaller atom to attract the shared electrons towards itself is greater than larger atom. So, electronegativity increases with decrease in atomic size.
(2) Oxidation state---
Electronegativity ∝
Higher oxidation state leads to greater electron attracting power, that is greater electronegativity. With increase in oxidation state atomic radius decrease, therefore electronegativity value increases.
Fe (1.80) < Fe2+ (1.83) < Fe3+ (1.96)
(3) Formal charge of an atom---
Electronegativity value of an atom in a compound is also affected by the formal charge on it. Electronegativity value increases with formal positive charge. Electronegativity value of nitrogen (N) in NH4+ is higher than that in NH3. Whereas electronegativity value decreases with formal negative charge. Electronegativity value of nitrogen (N) in NH2- is lower than that in NH3.
(4) Nature of hybridisation---
The overall tendency of any bonded atom to attract electron is directly influenced by its bonding environment. Electronegativity is the property of the orbital of an atom. The property of an orbital is influenced by its state of hybridisation.
The order of penetration of atomic orbitals are---
s > p > d > f
Thus, the effective nuclear charge on s orbital should be greatest. Hence hybrid orbitals with greater s character should be more electronegative than hybrid orbitals with less s character. The order of electronegativity of hybrid orbitals is---
sp > sp2 > sp3
(a) Compare the acidity of methane (CH4), ethylene (C2H4) and acetylene (C2H2)---
The hybridisation of central carbon of methane (CH4) is sp3, ethylene (C2H4) is sp2, and acetylene (C2H2) is sp. Since the order of penetration are s > p > d > f, the hybrid orbitals with greater s character should be more electronegative than hybrid orbitals with less s character.
Methane (CH4) : sp3 : 25% s character
Ethylene (C2H4) : sp2 : 33.3% s character
Acetylene (C2H2) : sp : 50% s character
Therefore, the order of acidity is---
CH4 < C2H4 < C2H2
Due to enhanced electronegativity of carbon (C) atom in C2H2 and C2H4 molecules the electron pair involved in the formation of C―H bond is shifted more towards C-atom (Cδ-―Hδ+). As a result, when C―H bond gets broken, H atom is obtained as H+ ion. So, the formation of hydrogen ion (H+) in C2H2 and C2H4 explain the acidic nature of these molecules. Due to less electronegativity of C atom in CH4 molecule the H atom remains neutral and CH4 does not show acid character.
(b) Compare the basicities of R―NH2, C5H5N and R―C≡N ---
The electronegativity of nitrogen (N) atom and the availability of N lone pair depends on its state of hybridisation. The electronegativity of N increases with increasing the s character in the hybrid orbitals and hence its basic character decreases.
R―NH2 : N is sp3 hybridised
C5H5N : N is sp2 hybridised
R―C≡N : N is sp hybridised
Therefore, R―NH2 is fairly basic, C5H5N is feebly basic and R―C≡N has no basic property. So, the order of basic character is---
R―NH2 > C5H5N > R―C≡N
(5) Nature of other atoms---
Electronegativity of an atom is influenced by the nature of other atoms with which it is attached. The electronegativity of carbon (C) atom is different in CH3I and CF3I. The C-atom is less electronegative than I-atom in CH3I molecule. This creates a partial positive charge on C-atom and partial negative charge on I-atom. So CH3I molecule represented as (CH3)+I-. On the other hand, in CF3I molecule, due to presence of highly electronegative F-atom, C-atom becomes more electronegative than I-atom. So CF3I molecule represented as (CF3)-I+.
Because of the difference in electronegativity of C-atom in CH3I and CF3I they hydrolysed in presence of OH- differently.
CH3+I- + OH- → CH3OH + I-
CF3-I+ + OH- → CF3H + OI-
(6) Ionisation energy and Electron affinity---
Mulliken proposed that the average of ionisation energy (IE) and electron affinity (EA) is proportional to the electronegativity (EN) of an atom---
EN ∝
So, the atom which have higher values of ionisation energy and electron affinity also have high values of electronegativity.











No comments:
Post a Comment