Super Phosphate
The three elements of vital importance for plant growth are nitrogen, potassium and phosphorus, the super phosphate being the principal source of the last element, that is phosphorous.
Super Phosphate of Lime
The artificially prepared mono calcium phosphate Ca(H2PO4)2 is known as super phosphate of lime. Commercial super phosphate is a mixture of mono calcium phosphate and crystalline calcium sulfate, that is---
Ca(H2PO4)2 + CaSO4 ∙ 2H2O
Super phosphate of lime contains about 16% P2O5. The soluble calcium phosphate prepared by treating rock phosphate with H2SO4 is called super phosphate of lime.
Ca3(PO4)2 + 2H2SO4 + 4H2O ---> Ca(H2PO4)2 + 2CaSO4 ∙ 2H2O
Manufacture of Super Phosphate of Lime
Raw materials: Rock phosphate of the composition 3Ca3(PO4)2, CaF2 is the starting material for manufacturing both forms of superphosphate. Strong H2SO4 (93-98) % is used in most plants.
Preparation process: Normal super phosphate is manufactured by mixing equal quantities of powered phosphate rock and chamber acid (specific gravity 1.45-1.60) into a cast iron mixture provided with a stirring mechanism. The mass stirred about 5 minutes and then it is allowed to remain for 1 day. Now the temperature rises to about 1000C-1100C as the reaction is exothermic. A mixture of fumes consists of HF (from CaF2), SiF4 (from CaF2 and silica) and CO2 (from lime stone) are evolved. These gases make the material porous.
As the reaction proceeds, the mixture stiffens and ultimately sets to a solid mass. After someday, it becomes perfectly dry. The gases are washed by spraying water in two successive towers. The resulting HF solution is then neutralized either by Na2CO3 or by NaF and finally treated with washed sand to form hexafluoro silicic acid. The latter is further neutralized by Na2CO3 to form sodium silico fluoride (Na2SiF6) or with magnesium to form magnesium silico fluoride (MgSiF6).
Reaction: The main reaction involved in the formation of super phosphate of lime is---
Ca10(PO4)6F2 +7H2SO4 + 3H2O ----> 2CaH4(PO4)2∙ H2O + 7CaSO4 + 2HF
[Ca3(PO4)2, CaF2] [Ca(H2PO4)2]
Na2SiF6 or MgSiF6 are useful by products. Super phosphate of lime is used principally as a fertilizer. It is water soluble fertilizer.
Triple Super Phosphate
Triple super phosphate or concentrated super phosphate contain about (44-47)% P2O5 which is nearly three times as in normal super phosphate.
Manufacture of Triple Super Phosphate
Raw materials: 78% H3PO4 (content 52-54% P2O5), powdered rock phosphate (35% P2O5).
Reaction: The reaction involved in the formation of triple super phosphate is---
CaF2,3Ca3(PO4)2+14H3PO4 = 10Ca(H2PO4)2+2HF
Preparation process: crushed rock phosphate is mixed with requisite quantity of phosphoric acid (H3PO4) of proper strength (strength is about 78%) in cast iron mixer, lined internally with lead, protected by acid proof bricks at 600C–700C. The mass is discharged with 20% moisture to a wet storage site, where the mass is allowed to age for about 30 days, and thereby the mixture is slowly proceeded to completion. The acid after the aging is disintegrated, dried at 2000C, sized and packed.
Type of rock and acid used in the manufactured process of triple super phosphate, and on the granular or non-granular nature of the product decided the properties of triple super phosphate.
Flow chart for the preparation of triple super phosphate
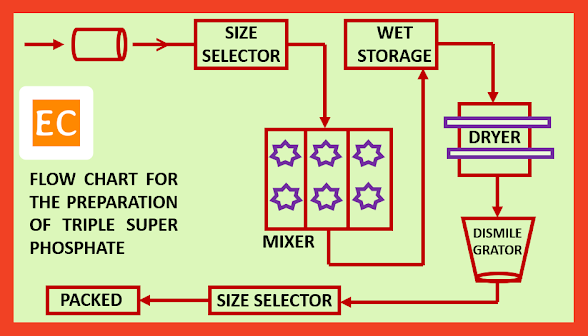
FLOW CHART FOR THE PREPARATION OF TRIPLE SUPER PHOSPHATE












No comments:
Post a Comment