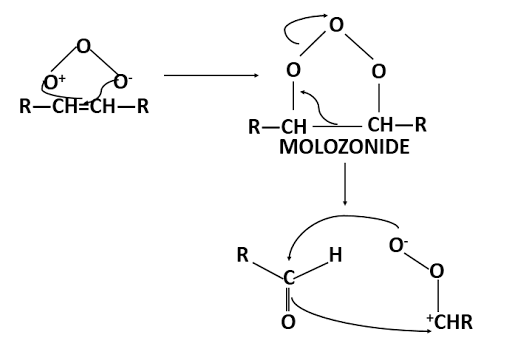
Alkenes add on ozone to form ozonides. The reaction proceeds via the formation of molozonide. Ozone is consider to behave as------O = O+— O- <------> O+ — O —O-
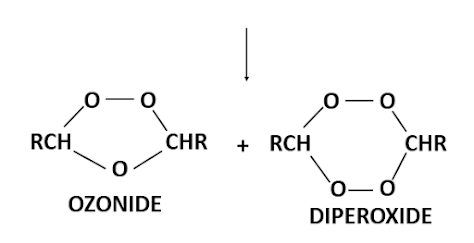
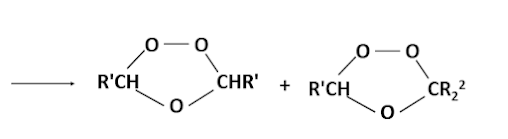
This mechanism of fission followed by recombination is supported by the fact that if the reaction is carried out in the presence of a ketone two ozonides are produced.
Furthermore if the alkene is unsymmetrical then two carbonyl compounds and two dipolar ions are possible and their combination can lead to three different ozonides. Also because the ozonides have cyclic structures, each one can exist in both cis and trans forms. Thus six ozonides can possible from alkene of the type R'R2C=CR3R4.
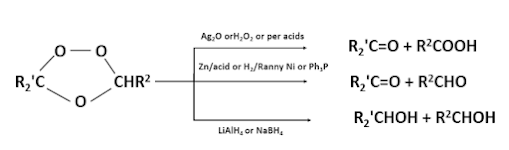
1.The ozonides is oxidised by means of Ag2O,H2O2 or
per acids there by producing acids and/or ketones.
2.(a)Reduction of the ozonides with Zn/acid, H2/Ranny Ni, Ph3P etc
gives aldehydes and or ketones.
(b) Reduction may also be carried out with LiAlH4, NaBH4 the products are the corresponding alcohols of the carbonyl compounds formed in (a).
The complete process of preparing the ozonides and decomposing it is known as ozonolysis, and is used to determining the position of a double bond in any olefinic compound.

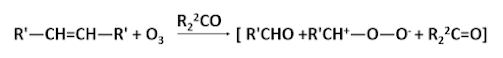










No comments:
Post a Comment