FRIEDEL CRAFTS REACTION
The carbon atom of alkyl halides Rδ+―Xδ- is electrophilic but is so weak that to effect the substitution of aromatic species. So, the electrophilicity of carbon may be enhanced by the addition of a species which is able to accept electrons from halogen, that is X and the reaction then occurs with less reactive aromatic compounds. This principle may be applied to the formation of bonds between aromatic and aliphatic carbon atoms by Friedel-Crafts alkylation and acylation.
ALKYLATION
Alkylating agents are halides, alcohols, esters, alkenes, aldehydes and ketones. Reactions of the first four classes are normally catalysed by Lewis acids and those of the last three by proton acids. In alkylation AlCl3 is employed as the catalyst. The order of effectiveness of the Lewis acid catalysts has been shown to be---
AlCl3>FeCl3>BF3>TiCl3>ZnCl2>SnCl4
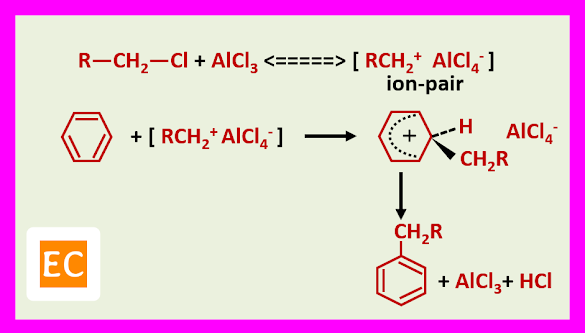
With primary and secondary halides, it is believed that an ion-pair is formed with the Lewis acid, the carbocation part of which then effects substitution.
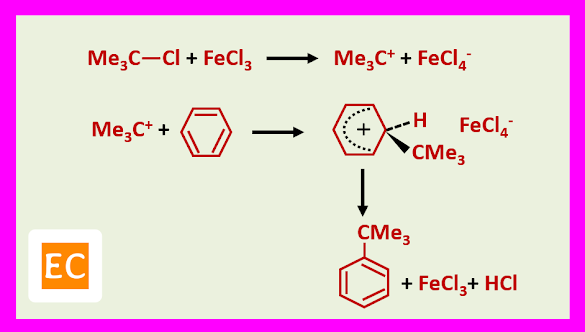
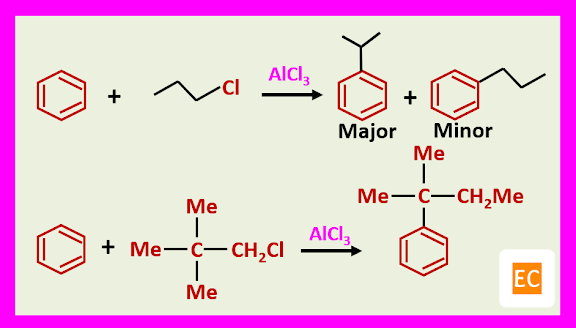
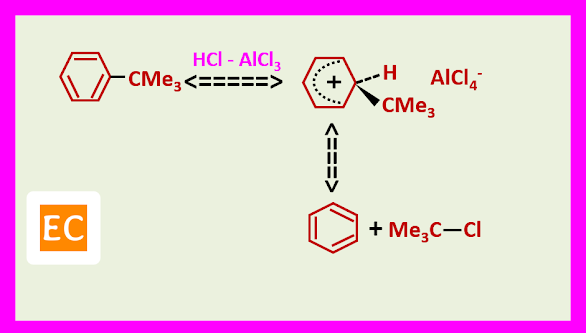
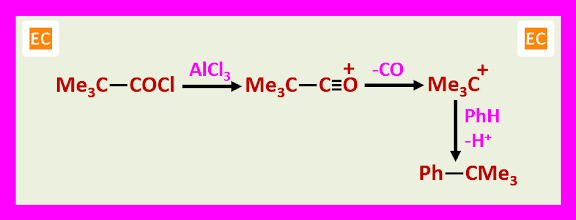
Free carbocations are thought to be involved with tertiary halides. For example, benzene tertiary butyl chloride, FeCl3 give tertiary butyl benzene in 80% yield via the tertiary butyl cation.
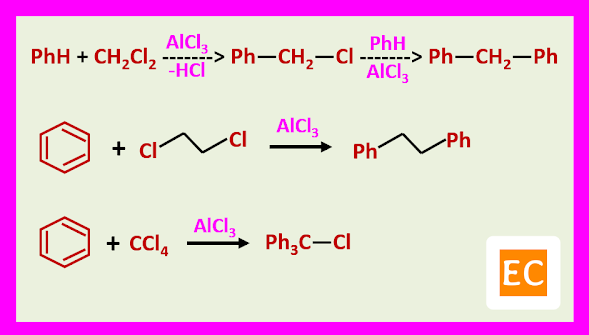
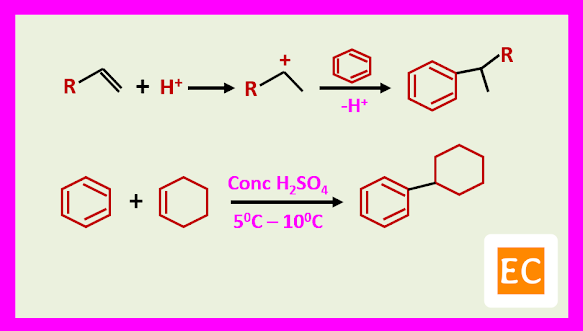
Some Examples:
REACTIVITY
Aromatic compounds whose nuclear reactivity is comparable with or greater than that of benzene can be successfully alkylated but strongly deactivated compounds do not react. For example, chloro benzene reacts but nitro benzene is inert. Phenol do not react satisfactorily because they react with the Lewis acids at oxygen (ArOH + AlCl3 ---> ArOAlCl2 + HCl) and the resulting compound is usually only sparingly soluble in the reaction medium so that it reacts slowly. Aromatic amines are not suitable for alkylations because aromatic amines form strong complex with Lewis acids.
PROBLEMS OF FRIEDEL CRAFTS ALKYLATION
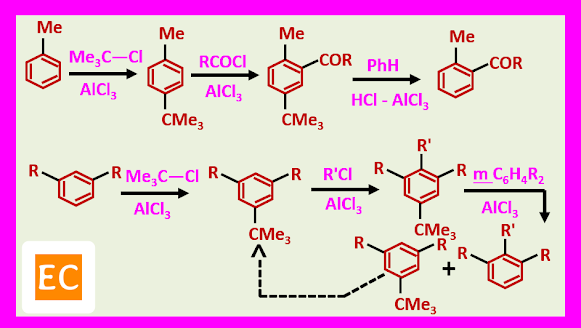
Since alkyl groups are activating in electrophilic substitutions, the product of alkylation is more reactive than the starting material and further alkylation occurs. For example, the methylation of benzene with methyl chloride in the presence of AlCl3 gives a mixture containing toluene, the xylenes, tri and tetra methyl benzenes, penta methyl benzenes and hexa methyl benzene.
Many alkyl groups rearrange during alkylation.
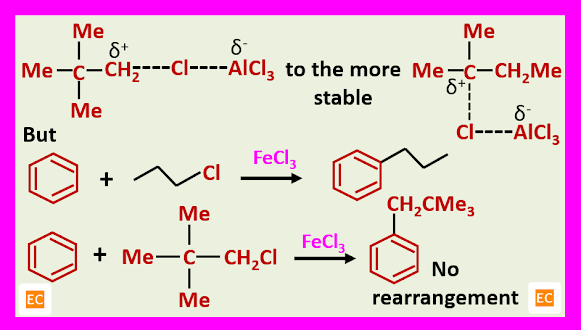
The reason probably comes from the fact that the initial electrophilic complex being polarized enough to allow the rearrangement.
The explanation is that the complex with the weaker Lewis acid, FeCl3 is not now polarized enough to allow the isomerisation taking place.
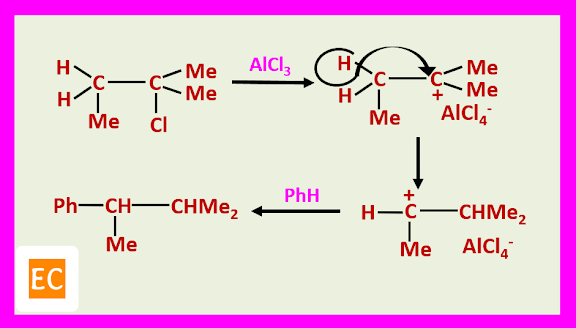
However, in some instances, during alkylation, rearrangement of tertiary halides occurs.
A probable explanation is that although the tertiary ion is the more stable of the two, it is also the less reactive, so that the faster reaction of the secondary ion dominates, even though this ion is present in smaller concentration.
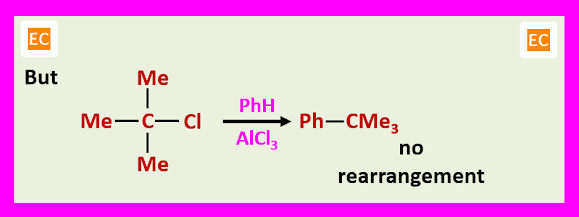
Tertiary butyl halides do not undergo rearrangement during alkylation, probably this would involve formation of the highly energetic carbonium ion.
SYNTHETIC UTILITY
Alkylation is reversible, so that the reaction is thermodynamically controlled. For example, a monosubstituted benzene usually gives mainly meta-alkyl derivative, since this is thermodynamically most stable.
Just as tertiary alkyl groups are the most readily introduced during alkylation, they are also readily removed by the reverse reaction, departing as the relatively stable tertiary carbocations.
This enables the tertiary butyl group to be used as a protective group, to protect the most reactive position, in a compound in order to effect reaction elsewhere.
ACYLATION
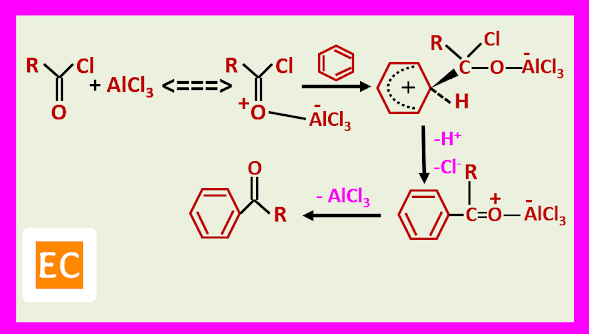
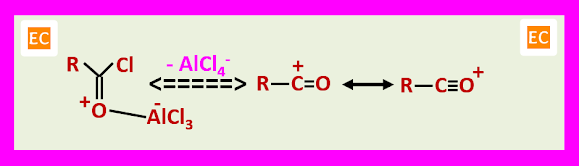
The acylation can be brought about by an acid chloride or anhydride in the presence of a Lewis acid or by a carboxylic acid in the presence of a protic acid. In the Lewis acid catalysed methods, two electrophiles are involved, one is an oxygen-bonded complex.
The other is an acylium ion.
There are a number of differences between acylation and alkylation.
1. In the alkylation does not require stochiometric quantities of the Lewis acid, because this is regenerated in the last stage of the reaction, but in case of acylation, requires greater than equivalent quantities because the ketone which is formed complexes with the Lewis acid.
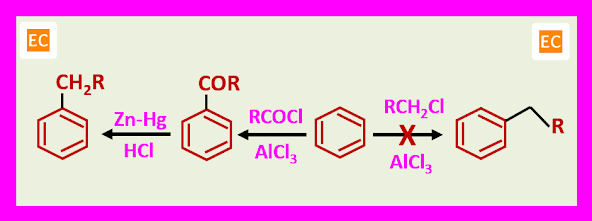
2. In the electrophilic substitution acyl groups deactivate aromatic nuclei, so the products of acylation are less reactive as compare to the starting materials and so the monoacylated product is easy to isolate. This makes acylation a more useful procedure than alkylation, and alkyl derivatives are often more satisfactorily obtained by acylation followed by reduction of carbonyl to methylene than by direct alkylation.
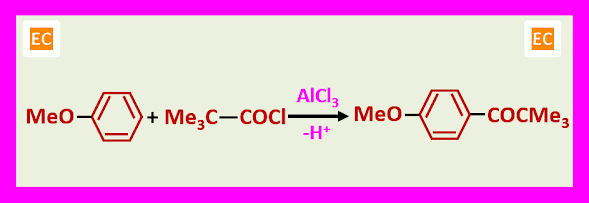
3. One limitation attempted acylation with derivatives of tertiary acids may lead to alkylation.
The driving force for the decarboxylation resides in the relative stability of tertiary carbocations. However, more reactive aromatic compounds can react before decarboxylation occurs.
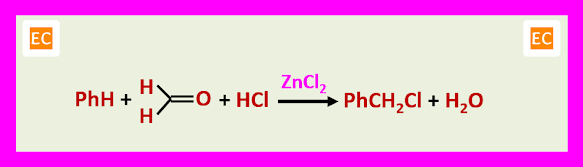
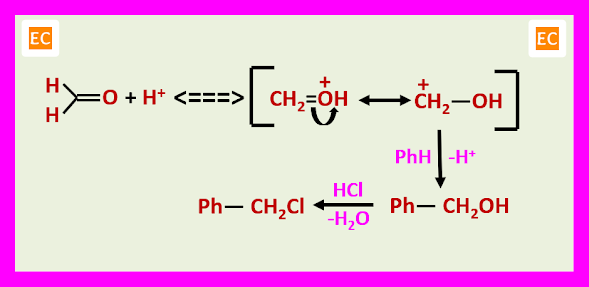
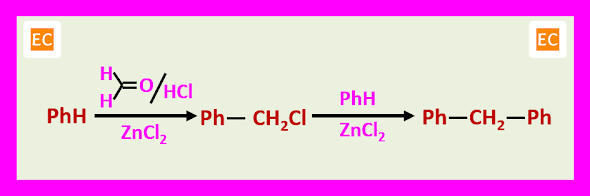
CHLOROMETHYLATION
With the appropriate halogen acid other halogen methylation such as fluoromethylation, bromomethylation and iodomethylation may be carried out.
The chloromethylated product can be alkylate another molecule of the aromatic compound in the presence of the acid catalyst.
This secondary reaction is of the particular significance when the aromatic compound is strongly activated and for this reason chloromethylation is not a suitable procedure for phenols and anilines.
The principal value of chloromethylation lies in the ease of displacement of benzylic chloride by nucleophiles. Conversion into the corresponding alcohols PhCH2OH, ethers PhCH2OR, nitriles PhCH2CN and amines PhCH2-NR2.