pH and pOH
Ion product of water
The pH and pOH
[H+][OH-] = 10-14
log[H+]+log[OH-]=log [10-14]= -14
pH + pOH = 14
[H+]>[OH-]
[H+]> 10-7M
[OH-]< 10-7 M
[OH-]> [H+]
[OH-]> 10-7M
[H+]< 10-7 M
[H+] = [OH-] = (10-14)1/2 = 10-7M
This is one of the best Blogs for free tutorials about various parts of chemistry, such as element chemistry, organic chemistry, inorganic chemistry, physical chemistry, analytical chemistry, environmental chemistry, industrial chemistry, biochemistry and much more. Here we discussed about theory, structure of molecules, mathematical problems, conceptual problem, question and answer. Which can be very helpful for your concept generation, problem solving ability in different field of chemistry.
[H+][OH-] = 10-14
log[H+]+log[OH-]=log [10-14]= -14
pH + pOH = 14
[H+]>[OH-]
[H+]> 10-7M
[OH-]< 10-7 M
[OH-]> [H+]
[OH-]> 10-7M
[H+]< 10-7 M
[H+] = [OH-] = (10-14)1/2 = 10-7M
CARBENE
A reaction in which both the groups or atoms are lost from the same carbon is called an α elimination reaction and results in the formation of carbene.
Carbenes can be defined as neutral divalent carbon intermediates in which a carbon is covalently bonded to two atoms and has two non bonded orbitals containing two electrons between them.
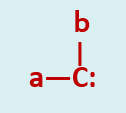 |
| Carbene |
The simplest member of the class is methylene a non-isolable species of molecular formula H2C:
Types of Carbenes
The two nonbonded electrons of a carbene may be either paired or unpaired. If they are unpaired the carbene is said to be in a triplet state, if they are paired then the carbene is called a singlet. One physical property that the singlet carbene is diamagnetic while the triplet is paramagnetic.
In the singlet state, a carbon atom is presumed to be approximate sp2 hybridization. Two of the three sp2 hybrid orbitals are utilized in forming two covalent bonds whereas the third orbital contains the unshared pair of electrons. The remaining p-orbital remains vacant.
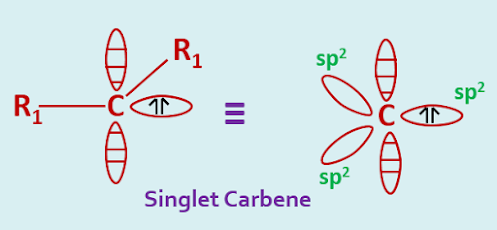 |
| Singlet Carbene |
The R1—C—R1angle would be expected to be contracted slightly from the normal 1200. The explanation comes from the valence bond electron pair repulsion theory, where l.p—l.p> l.p—b.p> b.p—b.p . Here the inter orbital repulsion will be greatest for the more diffuse lone pair orbital.
The carbon atom of a triplet carbene is sp hybridized. These two hybrid orbitals are involved in the bond formation with two groups and the remaining two electrons are placed, one each of the two unhybrid p-orbitals. These electrons have parallel spins.
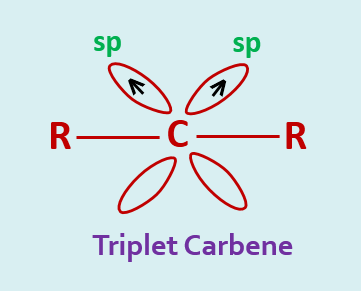 |
| Triplet Carbene |
Triplet carbene is more stable than the singlet. A triplet carbene is lying at about 8 K.cal/mole lower in energy than the singlet state.
Generation of Carbene
1. Photolysis of diazo compound :
Carbene is usually formed by photolysis of diazo compound when photolysis occurs in the liquid phase singlet carbene generated, but when photolysis occurs in gas phase triplet carbene generated.
 |
| Generation of Carbene by Photolysis |
2. From ketene :
Carbene can be generated from ketene, the reaction occurs in the presence of light in the gas phase.
R2C=C=O ---------> R2C: + CO
3. From alkyl halide :
The three mixed alkyl halide, such as CHF2Cl, CHF2Br, CHF2I undergo concerted elimination to carbenes.
OH- + CHF2Br --------> H2O
+ F2C: + Br-
4. From the reaction of chloroform and base :
When chloroform reacted with a base like K+O-CMe3 dichloro carbene is generated.
HCCl3 + K+O-CMe3
------> Cl3C- + Me3COH + KCl
Cl3C- ----> Cl2C: + Cl-
5. From esters of trichloroacetic acid :
When esters of trichloroacetic acid reacted with a base like MeO- dichloro carbene is generated.
Cl3CCOOEt +MeO- -------> Cl3C-
+ CH3OCOOEt
Cl3C- ----> Cl2C: + Cl-
6. From the reaction of carbon tetrachloride with alkyl lithium :
Butyl lithium and carbon tetrachloride react at low temperature (1000C) through halogen-metal interchange to give lithium trichloromethane. On warming (600C) this lithium salts yield dichloro carbene.
CCl4 + C4H9Li ------>
LiCCl3 + C4H9Cl
LiCCl3 -------> Cl2C: + LiCl
Reactions of Carbene
1.Cycloaddition reaction :
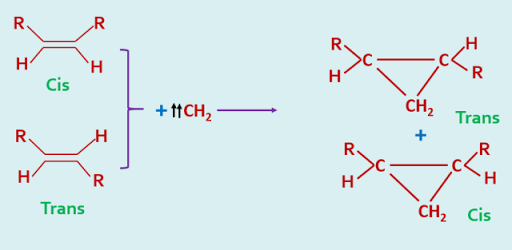
Singlet carbenes add to alkenes in a stereospecific manner i.e stereochemistry of the alkene is retained in the cyclopropane.
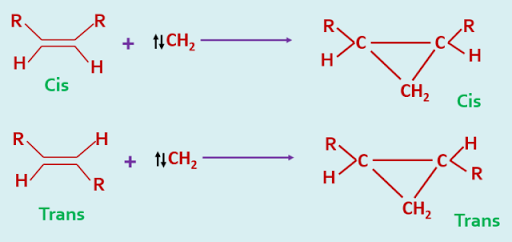 |
| Addition of Singlet Carbene |
In contrast, the addition of triplet carbenes to alkenes takes place with non stereospecificity.
 |
| Addition of Triplet Carbene |
The stereochemistry of these cycloaddition is so specific that it may be used as a diagnostic test for the identification of singlet and triplet carbenes.
The difference in behaviour between these two electronic states of a carbene has been explained in the following way. The addition of a singlet carbene to an alkene is a concerted process as there is no spin restriction on the simultaneous formation of two new sigma bonds of the cyclopropane, and hence it is a stereospecific process.
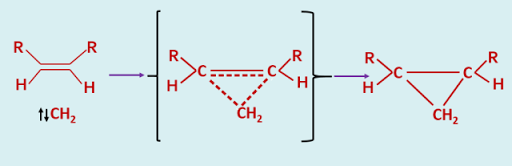 |
| Addition of Singlet Carbene - a concerted process |
In the case of triplet carbene, a cyclic transition state is not formed, the addition occurring stepwise as a radical fashion. This is represented as follows, the arrows indicating the direction of spin of each unpaired electron, and the assumption is made that the rotation about the single bond is more rapid than spin inversion.
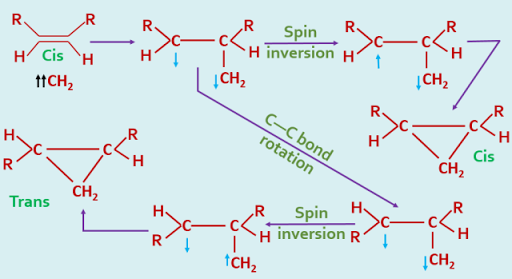 |
| Addition of Triplet Carbene - stepwise process |
2. Insertion reaction :
Frequently carbenes can be inserted into a C—H bond.
 |
| Insertion Reaction of Carbene |
The mechanism for insertion are recognized, it may be one step process or it may be two steps process.
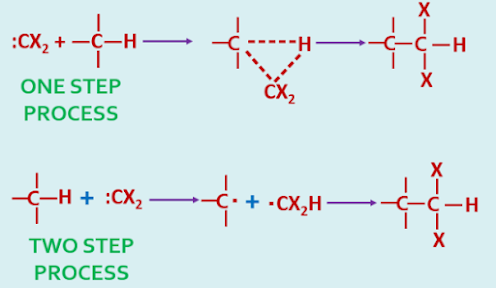 |
| Mechanism of Insertion Reaction of Carbene |
3.Simon-smith reaction :
Difficulty for the preparation of cyclopropane derivative using :CH2 and the solution is by using Simons-Smith reagent.
The addition of methylene, derived from diazomethane, ketene etc, to olifins is synthetically inefficient since insertion is a competing reaction.
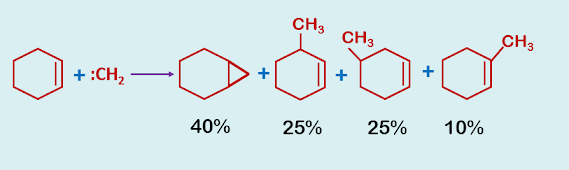 |
| Reaction of cyclohexene with carbene |
When an alkene is treated with methylene diiodide CH2I2,in presence of Zn-Cu couple we get a cyclopropane derivative.
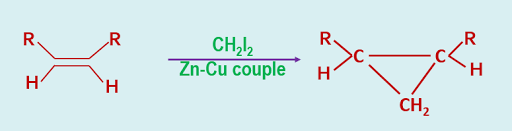 |
| Simon-Smith Reaction of Carbene |
This is known as Simon-Smith reaction.
Much of B—N chemistry relates that a BN unit isoelectronic with the CC unit. The electronegativity and atomic radius of carbon are intermediate between boron and nitrogen. Therefore there are many azoborane analogues of hydrocarbon in which a pair of carbon atoms replace B and N unit. Boron Nitride an important ceramic material that is isoelectronic with graphite. Boron Nitride has a similar sheet-like structure except that rings are aligned in an eclipsed fashion rather than staggered in graphite. Hexagonal Boron Nitride is colourless, electrically nonconducting, and is more resistant to chemical attack than graphite.
Boron reacts with N or NH3 on heating to form a binary nitride BN which is a white refractory solid. This compound is also obtained by fusing borax with NH4Cl.
Na2B4O5(OH)4 +
2NH4Cl -----> 2NaCl + 2BN + 2B2O3 + 6H2O
Pure Boron Nitride is best obtained by heating boron amide. This decomposes first to boron imide and finally into Boron Nitride.
2B(NH2)3 -----> B2(NH)3
+ 3NH3
B2(NH)3 ------> 2BN + NH3
Boron Nitride is also prepared by heating borazine--
B3N3H6 ------> 3BN + 3H2
2BN + 3H2O = B2O3 + 2NH3
2BN + 3F2 = 2BF3 + N2
BN + 4HF = NH4BF4
BN + KOH + H2O = KBO2 + NH3
HELIUM
CARBON NANOTUBES
Carbon nanotubes have seamless cylindrical structure contains one or more graphene layers, there are single wall carbon nanotube (SWCNT) and multi wall carbon nanotube (MWCNT), with open or closed ends. For perfect structure of carbon nanotubes the bonding of all carbons in carbon nanotubes are in hexagonal lattice except at their ends, other bonding patterns are pentagons, heptagons.The fundamental carbon nanotube is a single wall structure and best described as a rolled-up tubular shell of graphene sheet, the body of the tubular shell is made up of hexagonal rings (in a sheet) of carbon atoms, where as the ends are capped by a dome-shaped half fullerene molecules. Diameter of single wall carbon nanotube is 0.8 - 2 nm.The arrangement of the carbon atoms in the hexagonal network of the multi wall carbon nanotube is often helicoidal, resulting in the formation of chiral tubes. Multi wall carbon nanotube will typically be composed of a mixture of cylindrical tubes having different helicity or no helicity, thereby resembling turbostratic graphite. Diameter of multi wall carbon nanotube is 2 - 20 nm.
SYNTHESIS OF CARBON NANOTUBES
Carbon nanotubes can be prepared by varius ways, such as arc discharge, laser ablation, chemical vapor deposition (CVD), electrolysis, flame synthesis etc.
1. Arc Discharge ---
For the synthesis of carbon nanotubes this is one of the oldest method . 4-30 nm in diameter and 1 mm in length carbon needles were grown on the negative electrode used for the direct current (DC) arc discharge. A gas mixture of 10 Torr methane and 40 Torr argon filled in a pressurised chamber used during the process. Two thin electrodes installed vertically in the center of the chamber. The cathode, that is the lower electrode had a shallow dip to hold a small piece of iron during the evaporation. A DC current of 200 A and 20 V was used between the electrodes for the generation of arc. For the synthesis of single wall carbon nanotube (SWCNT) the use of three components such as methane, argon and iron was essential. As a result of arc discharge carbon soot was produced and carbon nanotubes grew in the negative cathode that contained a iron catalyst. The diameter of the nanotubes distributed between 0.7 nm to 1.65 nm.
2. Laser Ablation ---
A pulsed laser is made to strike at graphite target in the laser ablation process, in the presence of a inert gas such as helium in a high temperature reactor for vaporizes a graphite target. As the vaporized carbon condenses the nanotubes were developed on the cooler surfaces of the reactor. For the vaporization of target more rapidly a second pulse was used, that is in this process two step laser ablation was used. The benefit of two step laser process for minimised the carbon deposited as soot. In this method tubes grow on catalysts atoms and grows continued until to many catalysts atoms aggregate at the end of the tube. The produced carbon nanotubes diameter was 10 - 20 nm and length was 100 micron or more. By varying the reaction condition such as temperature, catalyst composition the average diameter and length of the produced carbon nanotubes could ve varied.
3. Chemical vapor deposition (CVD) ---
For the production of carbon nanotubes in large scale chemical vapor deposition (CVD) is a useful method. This method is very useful for controlling the growth of carbon nanotubes. In this process in the reaction chamber at a temperature of (700 0C – 900 0C) and at atmospheric pressure a mixture of hydrocarbon gases such as methane, ethylene, acetylene and process gases such as hydrogen, nitrogen, ammonia is made to react on heated metal substrate. Since hydrocarbon gas deposit and grows on metal catalysts (substrate), so carbon nanotubes was formed. On growing carbon nanotubes catalyst particles can stay at the bottom or on the top. The choice of metal catalysts and substrate were very important because they controlled the nature and type of carbon nanotubes. Generally silicon used as a substrate, sometimes aluminium even glass used as a substrate. Fe, Co, Ni metal nanoparticles were used as a catalyst. The diameter of the nanotube depends on the catalyst and substrate.
ULTRAMARINE BLUE Synthetic ultramarine blue is the most widely used blue pigment in the present time. Ultramarine blue pigment is ins...