ACID RAIN
When sulphuric acid, nitric acid
or carbonic acid in wet or dry forms (such as rain, cloud water, fog, hail,
sleet, snow, dew, dust) fall from atmosphere to the ground is termed as acid
rain or acid deposition or acid precipitation.
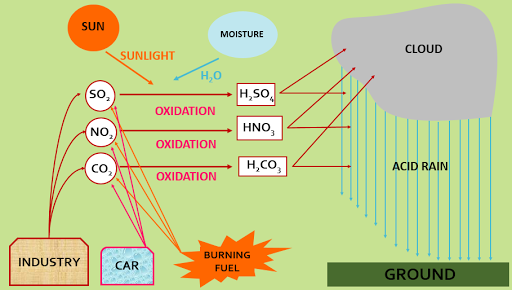
Causes of acid rain----
Sulphur di oxide (SO2),
carbon di oxide (CO2), nitrogen di oxide (NO2) [released
by industry sector, automobile sector (cars, buses, truck etc), or due to
combustion of fuels, eruption of volcanoes, burning of fossil fuels] are emitted
into the atmosphere and transported by wind and air currents.
1.Formation of sulphuric acid rain and its harmful effect----
In presence of sunlight SO2 dissociated photochemically as;
SO2------> SO (sulphur monoxide) + O (very reactive)
SO2+O ------> SO3
SO3 + H2O ------> H2SO4
H2SO4 forms in the above process mixed with rain water and falls on ground in the form of acid rain.
When this sulphuric acid rain falls over a historical monument, it gradually eats up the marble stone (CaCO3) of the monument and corrodes it slowly.
CaCO3 + H2SO4 ------> CaSO4 + CO2 + H2O
Due to corrosive nature of SO2 and H2SO4 the acid rain decolourises building materials such as lime stone, marble, roof slate and mortar. The corrosion of metals (Fe,steel,Zn,Al,bronze) is accelerared by acid rain.
2.Formation of nitric acid rain and its harmful effect---
NO2 present in the air mixed with moisture or O3 present in the atmosphere and forms HNO3.
4NO2 + O2 + 2H2O ------>
4HNO3
2NO2 +H2O +O3 ------->
2HNO3 + O2
Another process for the formation of HNO3 in the
atmosphere-
In presence of sunlight NO2 dissociated
photochemically as;
NO2 ---------> NO + O (very reactive)
NO2 + O ----> NO3
NO2 + NO3 -----> N2O5
N2O5 + H2O -----> 2HNO3
HNO3 forms in the above process mixed with rain water and falls on ground in the form of acid rain.
When this nitric acid rain falls over a historical monument,
it gradually eats up the marble stone (CaCO3) of the monument and
corrodes it slowly.
CaCO3 + 2HNO3 -------> Ca (NO3)2
+ CO2 + H2O
The acid rain fades the colour of the fabrics (cotton, nylon, rayon), lather and paper. The acid rain makes the water of lakes acidic and hence fish life comes in danger in this water. The yield of agricultural crops is also reduced.
3.Formation of carbonic acid and its harmful effect---
CO2 present in the air mixed with moisture
present in the atmosphere and forms H2CO3.
CO2 + H2O ----> H2CO3
H2CO3 forms in the above process mixed with rain water and falls on ground in the forms of acid rain.
Acid rain cause harmful effect on soil, the character of soil changed due to acid rain, the PH balance of soil affected. High altitude forests are especially vulnerable as they are often surrounded by clouds and fogs which are more acidic than rain. Plants, foods crops can also be damaged by acid rain. Acid rain caused harmful effect on fish and other living species that lives in water.











No comments:
Post a Comment