Nitrogen Fixation
Nitrogen fixation is a process by which nitrogen from atmosphere convert into ammonia by nature using bacteria such as Rhizobium, which is present in the nodules in the roots of legumes such as beans, peas etc. The process occurs at 0.8 atm pressure of N2 and at ambient temperature in Rhizobium bacteria and also some other independent bacteria. For the industrial synthesis of ammonia requires high temperature and pressure, and also requires iron oxide as catalyst and percentage of yield is only 15% to 20%, whereas the process of nitrogen fixation occurs at ordinary temperature and pressure. The process of nitrogen fixation is catalyzed by the metalloenzyme nitrogenase present in the bacteria. The reaction is as follows---
N2+8H++16MgATP+8e-->2NH3+H2+16MgADP + 16PO43-
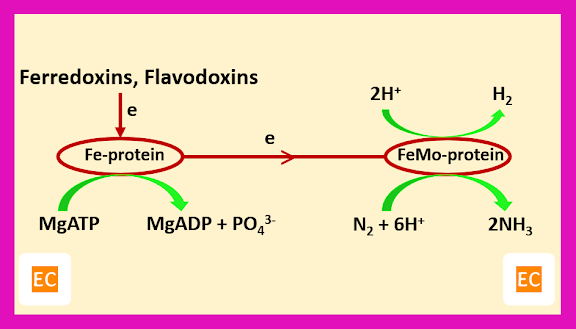
The ammonia produced in the process of nitrogen fixation is required for cell growth. The electrons required in this process, are supplied to metalloenzyme nitrogenase by reduced forms of ferredoxin and flavodoxins. Vanadium or iron or Fe-V or Fe-Fe or Fe-Mo may be present as a metal centres in the enzyme nitrogenase. The enzyme nitrogenase which is responsible for nitrogen fixation process contain two proteins. One protein is an iron protein (Fe-protein), whose molecular weight is about 60000, this protein contains an Fe4S4 cluster. Another protein is an iron molybdenum protein (Fe-Mo), whose molecular weight is about 220000, a tetramer containing two Mo atoms, and 30 to 32 Fe atoms together with sulphur. Mg-ATP (and MgADP) binds to the reduced form of the Fe-protein about 20 A0 from the Fe-S cluster. The Fe-proteins possible linked to the FeMo protein by salt bridge, transfer an electron for every two molecules of MgATP hydrolyzed. When enough electrons accumulate by FeMo protein (eight electrons are required for N2 conversion to 2NH3 by the enzyme nitrogenase because the reaction also produced H2) these are transfer to N2 with proton transfer from water.
Schematic representation of nitrogen fixation
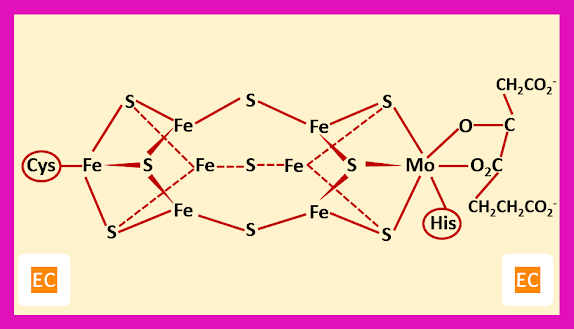
The FeMo-cofactor site of enzyme nitrogenase
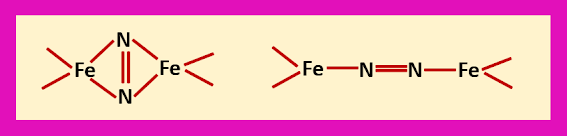
Two cuboidal fragments present in the cofactor, one is Fe4S3 fragment and other is Fe3MoS3 fragment. These fragments are linked to each other by sulphide bridges. The cluster is bound to the protein by a cysteine at one iron atom at the end and a histidine at the molybdenum atom at another end. This is a six coordinated molybdenum and the coordination number of molybdenum is fulfilled by the homocitrate ion, which is present at the extreme right side. During the enzyme turnover there is a little change in the coordination of Mo. At the interface of the two cube fragments the iron atoms have open coordination sites. These iron atoms may be served as the site for binding dinitrogen instead of Mo.
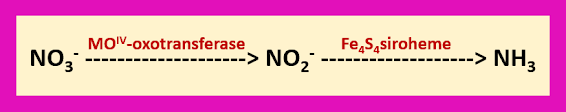
Another interesting point of this nitrogen fixation process is that formation of nitrates from lightning discharges. By using assimilatory nitrate and nitrate reductases, the nitrates are converted to ammonia.
The enzyme nitrate reductase is also containing molybdenum.
No attempts have been proved successful for the production of ammonia in industrial scale at ordinary temperature and pressure. It is still an unanswered question how the enzyme manages to carry out the reaction for the production of ammonia from atmospheric nitrogen by nitrogen fixation process at ambient temperature and less than 1 atm pressure of N2.











No comments:
Post a Comment