pH
and pOH
Ion product of water
In
the pure state, water is dissociated to a very small extent into hydrogen ion and hydroxyl ion, and behaves as a weak electrolyte. The
equilibrium constant of the dissociation, is given by ----- H2O
<=====> H+ + OH-
K =
[H+][OH-] / [H2O]
K X [H2O]
= [H+][OH- ]
In any dilute aqueous solution, the concentration of the water molecules,(= 55.5 moles/litre) greatly exceeds that of any other ion, [H2O] can be taken as a constant. Hence,
K X [H2O]
= Kw =
[H+][OH- ]
Where Kw, water dissociation constant (ionic product of water). The [H+] ion concentration in pure water is 1 x 10-7 . Since [H+] = [OH-] in pure water, therefore-
Kw
= [H+][OH-]
= [1x10-7
x1x10-7]
= [1 x 10-14]
A strong acid such as hydrochloric acid or nitric acid when added to water, the concentration of hydrogen ion increases very high and at that time the concentration of hydroxyl ion becomes very low, so that the product of concentration of hydrogen ion and hydroxyl ion remains constant, similarly when strong base such as sodium hydroxide or potassium hydroxide added to water the concentration of hydroxyl ion increases very high and at that time the concentration of hydrogen ion becomes very low, so that the product of concentration of hydrogen ion and hydroxyl ion remains constant.
The pH and pOH
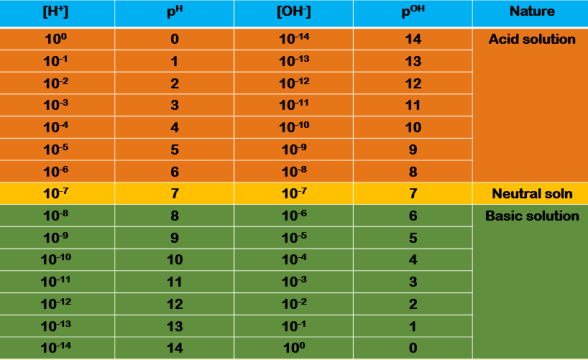
An aqueous solution whether it is in acidic condition, alkaline condition or in neutral condition it always contains hydrogen ion and hydroxyl ion. The condition of acidic and alkaline depends upon which ion concentration greater than other, If the concentration of hydrogen ion is greater then the solution is acidic condition and if the concentration of hydroxyl ion is greater then the solution is alkaline. Knowing the concentration of one ion other can be calculated, but it is conveinent to express acidity or alkalinity of a solution by reffering to the concentration of hydrogen ions only. Now hydrogen ion concentration can vary within wide limits, usually from about 1 mole per litre (as in 1 M HCl) to about 10-14 mole per litre (as in 1 M NaOH). Sorensen therefore introduced a term pH for indicating hydrogen ion concentration which is defined as : The negative of the logarithm of [H+] ion concentration was defined as pH.
pH
= -log [H+]
Or,[H+]
= 10-
pH
Thus if a solution has hydrogen ion concentration 10-1 M its pH is 1, and if hydrogen ion concentration is 10-14 M its pH is 14. For such solution having [H+] ion concentration in the range 10-1 M to10-14 M it is more convenient and meaningful to express acidity in terms of pH rather than ion concentration. As a result, the use of small fractions or negative exponents can thus be avoided. In the case of monobasic acid molarity and normality are the same but in the case of polybasic acid molarity and normality are different. Thus 0.1M H2SO4 is equivalent to 0.2 N H2SO4, so that : pH
= - log [H+]
= - log (0.2)
= 0.699
The negative of the logarithm of [OH-] ion concentration was defined as pOH.
pOH
= - log [OH-]
Or,[OH-] = 10-pOH
From the above two relations, we know that the lower the pH, the more acidic the solution is. If the acidity of the solution goes down to 10 fold its pH goes up by 1 unit. For example, if a solution has pH 1, its [H+] ion concentration is 10 times than for a solution whose pH is 2. Taking the case of [OH-] ion concentration, the pOH will go down by 1 unit (from 13 to 12). Recalling that the product of [H+]
and [OH-] is 10-14 and, therefore we can write the equation as :
[H+][OH-] = 10-14
log[H+]+log[OH-]=log [10-14]= -14
pH
+ pOH
=
14
On the basis of [H+] ion concentration and [OH-] ion concentration, we can differentiate between acidic, basic and neutral solution on one hand and on the basis of pH on the other hand.
An acidic solution is one in which [H+]
ion concentration exceeds that of [OH-] ion concentration.
[H+]>[OH-]
[H+]>
10-7M
[OH-]<
10-7
M
A basic solution is one in which [OH-] ion concentration exceeds that of [H+]
ion concentration.
[OH-]>
[H+]
[OH-]>
10-7M
[H+]<
10-7
M
A neutral solution is one in which [H+]
ion concentration and [OH-]
ion concentration are equal.
[H+]
= [OH-]
= (10-14)1/2 = 10-7M
In terms of pH we have the following relations :
The scale of pH :
 |
| The scale of pH |

No comments:
Post a Comment