PHOSPHAZENES OR PHOSPHONITRILIC HALIDES
PREPARATION OF PHOSPHAZENES
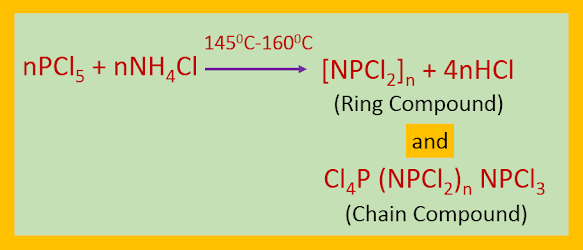
When PCl5 is refluxed with NH4Cl in presence of C2H4Cl2 or C6H5Cl, or heated with solid NH4Cl at 1450C–1600C, ring compounds of the type [NPCl2]n where (n = 3-8) and chain compounds of the type Cl4P(NPCl2)nNPCl3 are produced. The newly synthesized compounds are called phosphazenes or phosphonitrilic halides.
The bromo derivative is obtained by heating PBr5
with NH4Br and the fluoro derivative is obtained from the chloride on
treatment with NaF in acetonitrile.
[NPCl2]n + 2n NaF -----> [NPF2]
+ 2n NaCl
Alkoxy groups can be introduced in the polymer by replacing
Cl.
[NPCl2]n + 2n NaOR -----> [NP(OR)2]n
+ 2n NaCl
The iodide derivatives are not yet known. The rings compounds where n=3 and n=4 are stable and can be prepared by under controlled conditions.
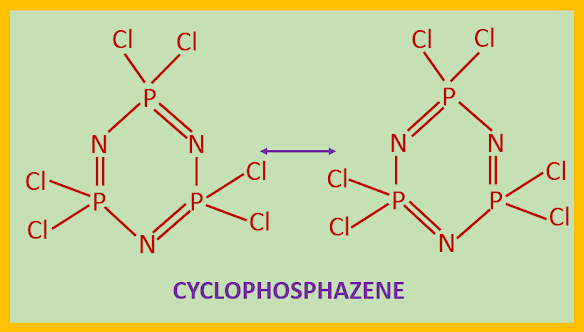
STRUCTURE OF PHOSPHAZENES
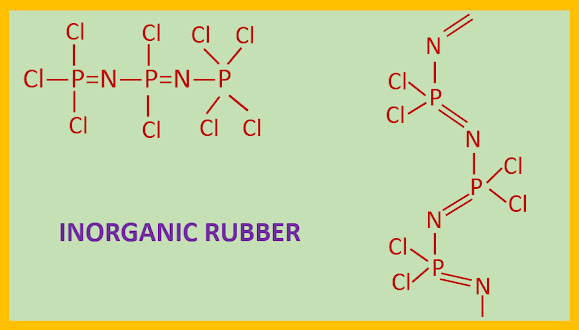
INORGANIC RUBBER
PROPERTIES OF PHOSPHAZENES
REACTIONS OF PHOSPHAZENES
[NPCl2]3 + 6CH3MgI ------>
[NP(CH3)2]3 + 3MgCl2 + 3MgI2
[NPCl2]3 + 6C6H5Li
------> [NP(C6H5)2]3 + 6LiCl
[NPCl2]3 + 6NaOR ------> [NP(OR)2]3
+ 6NaCl
[NPCl2]3 + 6NaSCN ------> [NP(SCN)2]3
+ 6NaCl
[NPCl2]3 + 6H2O ------>
[NP(OH)2]3 + 6HCl
[NPCl2]3 + 6NH3 ------>
[NP(NH2)2]3 + 6HCl
The lone pair of electrons present on each N atom in [NPCl2]3
molecule makes it basic and hence it forms adduct with Lewis acids like HClO4,
SO3, AlCl3 etc.
[NPCl2]3 + 2AlCl3 ------>
[NPCl2]3•2AlCl3
[NPCl2]3 + 3SO3 ------->
[NPCl2]3•3SO3
[NPCl2]3 + 2HClO4 ------>
[NPCl2]3•2HClO4