ESSENTIAL AMINO ACIDS
Amino acids, as their name implies are compounds that contains both an amino group and a carboxylic acid group. The general structure of amino acid is---
R―CH(NH2) ―COOH
Where, R is the side chain, it varies from amino acid to amino acid. In this structure of amino acid, the ―NH2 group is present at α position, located next to ―COOH group. So, it is the general structure of α-amino acids.
The general structure of β-amino acids is---
R―CH(NH2) ―CH2―COOH
Where, the ―NH2 is present at β position.
The general structure of γ-amino acids is---
R―CH(NH2) ―CH2―CH2―COOH
Where, the ―NH2 group is present at γ position.
The amino acids are particularly important because they are the monomeric units of biologically important polymers called peptides. Proteins are simply large peptides. Peptides and proteins serve many important roles in biological system, e.g., all enzymes are proteins. Hydrolysis of protein by acids, alkalis or enzymes, yield a mixture of amino acids. Mixture of amino acids obtained by the hydrolysis of proteins are mostly α-amino acids.
Twenty α-amino acids, however are known by widely accepted traditional names. These are the amino acids that occurs commonly as constituents of most proteins. The amino acids can be grouped according to the nature of their side chains.
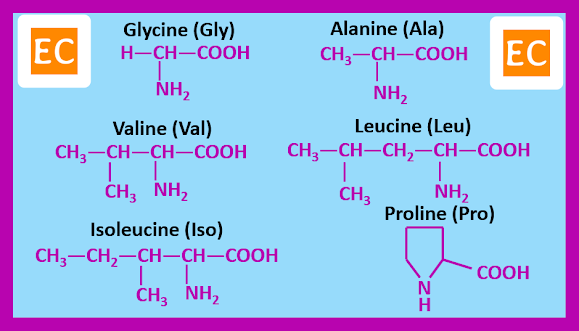
Amino acids with side chain containing H or aliphatic hydrocarbon
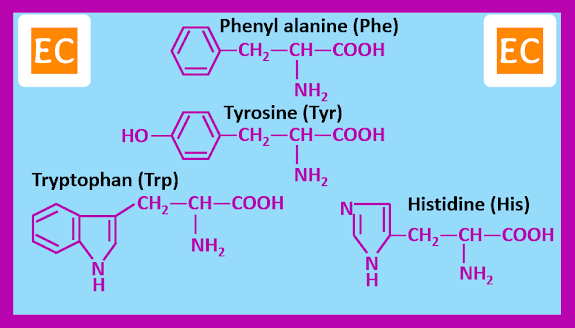
Amino acids with side chains containing aromatic groups
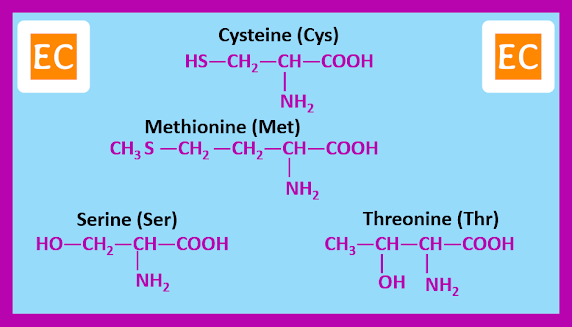
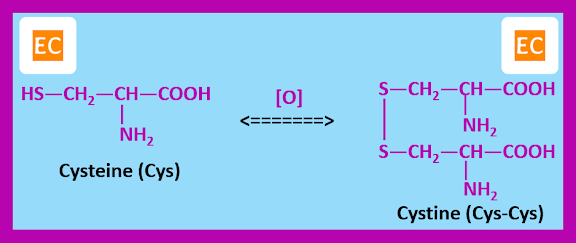
Amino acids with side chains containing ―SH, ―SCH3, or ―OH groups
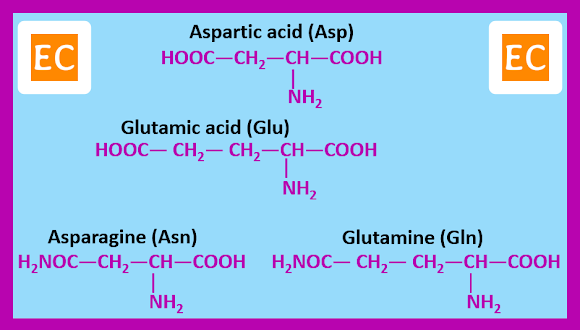
Amino acids with side chains containing carboxylic acid or amide groups
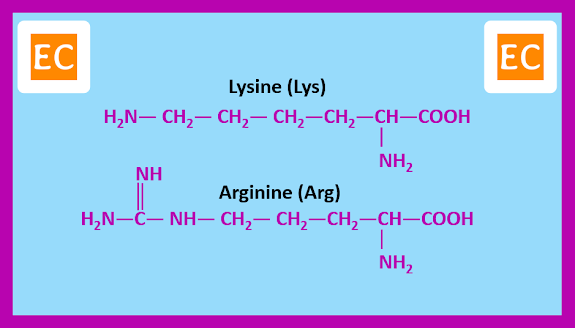
Amino acids with basic side chains
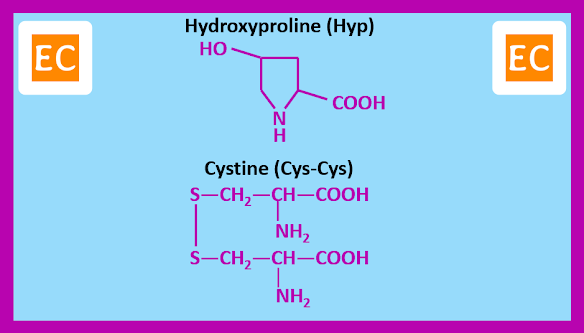
In addition to the above twenty α-amino acids there are two other α-amino acids
Configuration of natural amino acids
Zwitter ionic structures of amino acids---
NH3+―CH(R)―COO-
Evidences for the formation of Zwitter ionic structure of amino acid---
1. High melting point of amino acids in comparison to amines or carboxylic acids.
Ph―CO―NH―CH2―COOH this is N-benzoyl glycine (hippuric acid), its melting is 1900C.
The melting point of glycine (H2N―CH2―COOH) is 2620C.
2. Amino acids are insoluble in polar aprotic solvent such as ether. Most amines and carboxylic acids on the other hand, dissolve in ether. Although the water solubility of the different amino acids varies, all are highly soluble in water.
3. The dipole moment of the amino acids is very large, much larger than those of similar sized molecules with only an amine or a carboxylic acid group.
Dipole moment of propanoic acid (CH3―CH2―COOH) is 1.7 D (µ = 1.7 D).
Dipole moment of n-butyl amine (CH3―CH2―CH2―CH2―NH2) is 1.4 D (µ = 1.4 D).
Whereas, dipole moment of glycine (H2N―CH2―COOH) is 14 D (µ = 14D).
Obviously, a large dipole moment is expected for the molecules that contain a great deal of separated charge.











No comments:
Post a Comment