NITROLIM or CALCIUM CYANAMIDE
Nitrolim or calcium cyanamide is one of the most important fertilizers in the present day, contain about 20% of nitrogen. The formula of nitrolim or calcium cyanamide is CaNCN or CaCN2 and it is represented as N≡C―N=Ca or [(Ca2+) (-N=C=N-)], it is a derivative of cyanamide, N≡C―NH2. Nitrolim is grey powdered fertilizer.
When limestone means calcium oxide (CaO) and coke (C) heated in an electric furnace at about 20000C, calcium carbide (CaC2) is formed.
At 20000C, CaO + 3C ------> CaC2 + CO
When this calcium carbide (CaC2) treated with nitrogen (N2), calcium cyanamide or nitrolim is formed, this reaction is highly exothermic.
CaC2 + N2 -------> CaNCN + C ΔH = - 72.7 Kcal
Some important considerations during this manufacturing process are---
Temperature: Since the reaction is exothermic, so according to Le-chatelier principle, this reaction is highly favorable at low temperature, but this off set the activation energy of the reaction.
Activator: CaF2 is used as an activator to overcome the above difficulty. At low temperature, CaF2 activates the process of the reaction at a reasonably in speed.
Fixation: Nitrogen (N2) fixation limit cannot pass over 85% limit.
Raw Materials
Raw materials required for the manufactured of nitrolim or calcium cyanamide are calcium carbide (CaC2) [which is prepared from lime stone or calcium oxide (CaO) and coke (C)] and nitrogen (N2).
Manufacturing Process
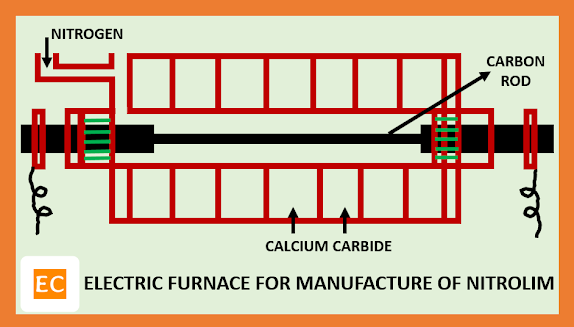
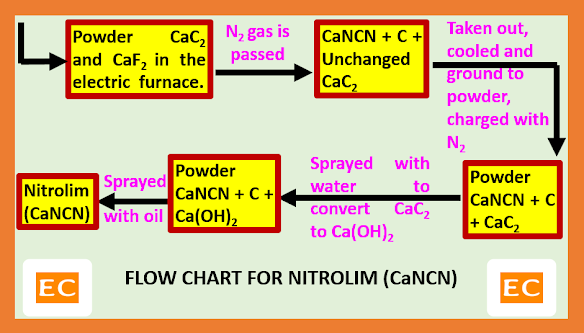
Powdered calcium carbide (CaC2) along with CaF2 (activator) are taken in the electric furnace fitted with pipe to get supply of nitrogen (N2). In the electric furnace, the central carbon electrode is connected to high voltage mains. When the temperature of the electric furnace reaches to 9000C, highly exothermic reaction leading to the formation of the calcium cyanamide or nitrolim. Allowing the reaction to continue by its own liberated heat at about 40-60 hours for completion. During this process temperature is maintained at about 10000C. After completion the reaction solid chank are taken out, and then disintegrated, powdered. Now first water is sprayed over the powdered to destroy any residual carbide and then spray oil. The grey powdered thus obtained is suitable for use.
Uses of Nitrolim or Calcium Cyanamide
Nitrolim or calcium cyanamide is used as a fertilizer due to its slow conversion into ammonia (NH3) and nitrate (NO3-). So, it is a good fertilizer as its effects are prolonged nature.
Action of Calcium Cyanamide or Nitrolim as Fertilizer
In the soil, when finely powdered nitrolim is applied, it gets hydrolyzed to calcium hydroxide [Ca(OH)2] and urea (H2N―CO―NH2). This hydrolysis process is catalysed by soil catalysis or microorganisms, which are present in the soil.
CaCN2 + 4H2O ------> Ca(OH)2 + CO(NH2)2 + H2O
Or, CaCN2 + 3H2O ------> Ca(OH)2 + CO(NH2)2
The urea formed in this process, is then hydrolysed with the help of enzyme urease into ammonia. The ammonia is then converted to nitrates by oxidation with the help of enzyme of bacteria.
CO(NH2)2 + H2O ------> CO + 2NH3
2NH3 + 3O2 -----> 2NO2- . 2H2O + 2H+ + Energy
2NO2- + O2 -------> 2NO3- + Energy











No comments:
Post a Comment