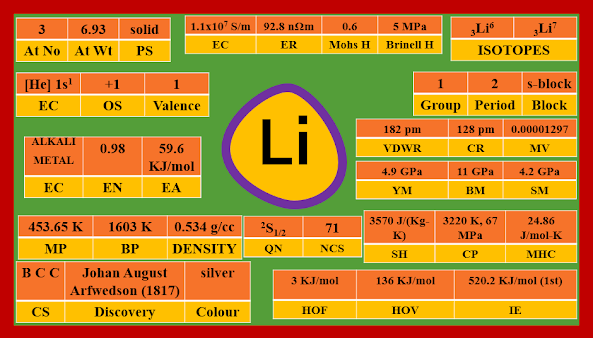
LITHIUM
Symbol --- Li
Abundance --- 0.0017% in earth's crust.
Physical state --- solid
Elemental Category --- Alkali metal
Colour --- silver
Discovery --- Johan August Arfwedson (1817)
Atomic no --- 3
Atomic weight --- 6.93
Period --- 2
Group --- 1
Block --- s-block
Known Isotopes--- 3Li3 3Li4 3Li5
3Li6 3Li7 3Li8
3Li9 3Li10 3Li11
3Li12
Stable Isotopes --- 3Li6 3Li7
Isotopic abundance --- [3Li6 (7.59%), 3Li7
(92.41%)]
Melting Point --- 453.65 K (180.50C)
Boiling Point --- 1603 K (13300C)
Density --- 0.534 g/cc
Electron Configuration --- [He] 2s1
Oxidation State --- +1
Valence --- 1
Electronegativity --- 0.98
Electron Affinity --- 59.6 KJ/mol
Ionisation Energy --- 520.2 KJ/mol (1st), 7298.1 KJ/mol (2nd), 11815 KJ/mol (3rd)
Covalent Radius --- 128 pm
Van der Waals radius --- 182 pm
Crystal Structure --- B.C.C (Body Centered Cubic)
Heat of fusion --- 3 KJ/mol
Heat of vaporisation --- 136 KJ/mol
Critical Point --- 3220 K, 67 MPa (661.239 atm)
Molar heat capacity --- 24.86 J/mol-K
Specific heat --- 3570 J/(Kg-K)
Thermal conductivity --- 84.8 W/(m k)
Molar volume --- 0.00001297
Speed of sound --- 6000 m/s
Magnetic type --- paramagnetic
Mass magnetic susceptibility --- +2.56x10-8 m3/kg
Molar magnetic susceptibility --- +
1.78 x 10-10 m3/mol
Lattice angles --- π/2, π/2, π/2
Lattice constants --- 351 pm,351 pm,351 pm
Quantum numbers --- 2S1/2
Neutron cross section --- 71
Electrical conductivity --- 1.1 x 107 S/m
Electrical resistivity --- 92.8 nΩ m
Mohs hardness --- 0.6
Brinell hardness --- 5 MPa
Young's modulus --- 4.9 GPa
Bulk modulus --- 11 GPa
Shear modulus --- 4.2 GPa











No comments:
Post a Comment