MERCURIAL DIURETICS
In the sixteenth century Paracelsus used calomel as a diuretic. In the year 1919 antisyphilitic agent, merbaphen, was tested for its diuretic action. In the mercurial diuretics Hg2+ is essentially present in the organic molecule. The general structure of most mercurial diuretics contains a chain of at least three carbon atoms and one mercury atom. The general structure of mercurial diuretics is as follows---
Y―CH2―CH(OCH3) ―CH2―Hg―X
Where,
X = Halide, OH, heterocyclic compound
Y = substituted side chain, substituted aromatic compound.
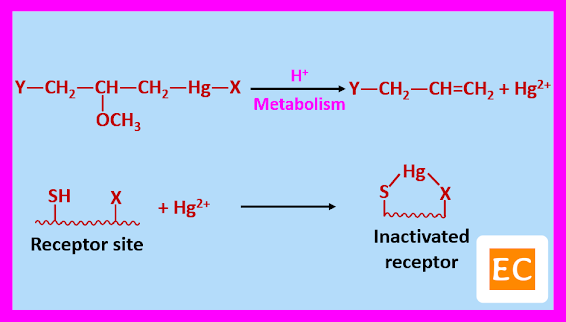
The Y group that is substituted side chain or substituted aromatic compound is hydrophilic in nature, which determines the distribution and rate of excretion of the compound. Whereas the X group that is halide or OH or heterocyclic compound affects the toxicity of the mercurial diuretics compound, irritation at the site of injection, and rate of absorption. The mercuric ion (Hg2+) of the mercurial diuretics dissociates and binds to the sulfhydryl enzymes and inactivating them in an acidic environment. Due to this reabsorption of sodium ion (Na+) is diminished and increased the excretion of sodium ion (Na+) and chloride ion (Cl-). More Cl- ion is lost than Na+ ion. As a result, cations such as hydrogen ion (H+) and lesser degree of potassium ion (K+) are also lost to maintain the electrical neutrality. Due to excess excretion of chloride ion (Cl-), bicarbonate (HCO32-) remains to maintain balanced anions, and the resulting picture is a hypochloremic alkalosis. Mercurial diuretics usually inhibit active chloride transport in the ascending limb of the loop of Henle.
The mercurial diuretics are now of limited use due to their pronounced and marked side effects such as renal tubular damage, mercurialism, hypersensitivity and excessive diuresis which may led to electrolyte depletion and vascular complications. Most of the mercurial diuretics are administered by intramuscular route. Some examples of mercurial diuretics are chlormerodrin, mercaptomerin sodium, meralluride, merethoxylline procaine, mercumatilin sodium etc.
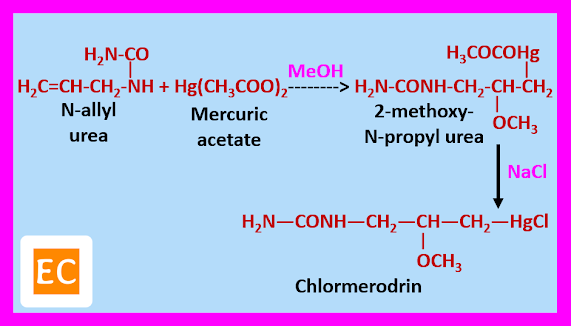
Synthesis of Chlormerodrin
It is prepared by the reaction of N-allyl urea with mercuric acetate in presence of methanol, as a result 2-methoxy-N-propyl urea is formed. When 2-methoxy-N-propyl urea react with NaCl chlormerodrin is produced.
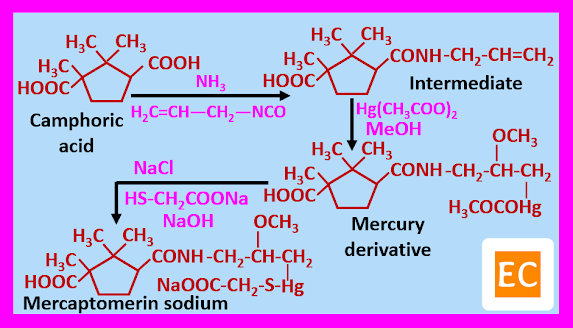
Synthesis of Mercaptomerin Sodium
Camphoric acid on reaction with ammonia in the presence of allyl isocyanate formed an intermediate, which on treatment with mercuric acetate in presence of methanol gives mercury derivative as acetate, which on reaction with sodium thioglycolate in presence of sodium chloride and aqueous sodium hydroxide produced mercaptomerin sodium.











No comments:
Post a Comment