BUNA - S or Styrene-Butadiene Rubber
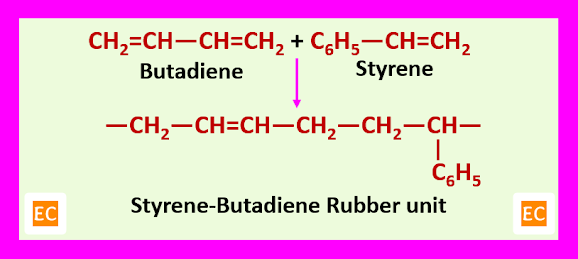
Buna – S or styrene-butadiene rubber is made by emulsion co-polymerization of butadiene and styrene, usually in the ratio of 3:1 in presence of a free radical catalyst at about 50C for 12-15 hours.
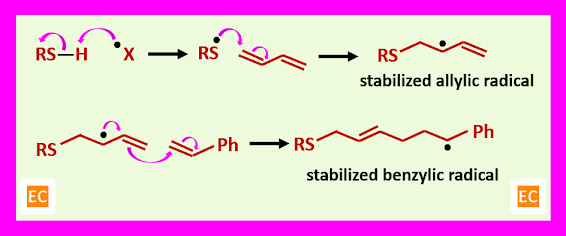
A material that is very like natural rubber is produced by radical co-polymerization of styrene and butadiene. A one-electron oxidizing agent acts as an initiator and a thiol (RSH) is used to start the polymerization process. The ratio of butadiene: styrene in the mixture is about 3:1, so there are no long runs of one monomer in the product. Butadiene is used as a starter unit. An allylic radical is produced as a first radical which is stabilized by conjugation with the remaining alkene in the old butadiene molecule. This radical is now added to another butadiene or to styrene. As a result, a benzylic radical is produced with the more stable trans double bond. Finally, a random co-polymer is produced which contains about 3:1 butadiene to styrene with mostly E -alkenes. Buna – S or styrene butadiene rubber is used in tyres and other applications where a tough and flexible rubber is needed.
Manufacture of Buna-S or Styrene-Butadiene Rubber
The production of starting materials are---
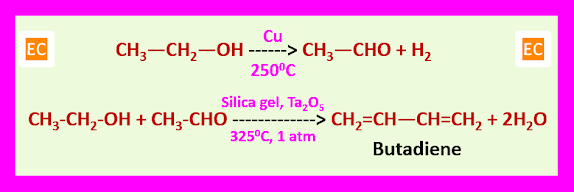
Butadiene: The main starting material is a gas at ordinary temperature. It is manufactured either from ethyl alcohol or obtained as a petroleum product.
Styrene: Styrene is manufactured by Daw process. In this process benzene mostly free from sulphur (thiophene) is dried azeotropically, and ethylene gas is passed through it at 850C-900C and 5 psi pressure in presence of AlCl3 acting as a catalyst.
C2H4 + C6H6 ---> C6H5―CH=CH2
Production process: Buna-S or styrene-butadiene rubber is manufactured by emulsion co-polymerization of butadiene and styrene. Two process such as hot process and cold process have been developed, depending on the reaction temperature. At the present time about 75% of the total production is carried out by the cold process, because of the formation of better and good quality by lowering the reaction temperature.
In the cold process, which gives better results at lower temperature, reducing agents are used. Free radical is obtained from the reducing agents such as thiol (RSH), benzoyl peroxide or sodium persulphate etc. The free radical helps the partial polymerization. The free radical is thus obtained react with the monomers and remain incorporated in the final product. The composition is butadiene 72%, styrene 28%, tertiary dodecyl mercaptan 0.17%, potassium resin soap 4.5%, sodium hydroxide 0.2%, ferrous sulphate hydrate (FeSO4 ∙ 7H2O) 0.14%, cumene hydroperoxide 0.15%, sodium phenyl naphthyl amine 1.25% and water 200%.
Tertiary dodecyl mercaptan is used as chain modifier and N-phenyl-2-naphthyl amine is used as antioxidant. The emulsion co-polymerization of butadiene and styrene in presence of a free radical catalyst takes place at about 50C for 12-15 hours. Since much heat is liberated during the process, soap in water emulsion is added to control the temperature of the reaction. A latex type of rubber is thus obtained. After the 90% copolymerization is completed, the latex and the monomers run in a blow down pressure vessel and treated with an inhibitor, in order to arrest the reaction. The latex is then made free from the monomers. Modifiers, such as alkyl hydrosulphides control the size of polymer molecules. The monomer free latex is thus obtained is treated with an anti-oxidant and then it is coagulated from the emulsion by NaCl and H2SO4 solution (1.8-2 PH). Unreacted butadiene and styrene are recovered and reused. Both 1,2 and 1,4 addition of styrene to butadiene occur, the later predominating.
In cold process, the chain length of the copolymer is less. Hence rubber is more elastic, less hard and more resistant. Styrene-butadiene rubber is more resistant to abrasion and weathering than natural rubber. Its toughness may be further reduced by adding some extenders, such as petroleum-based oils.