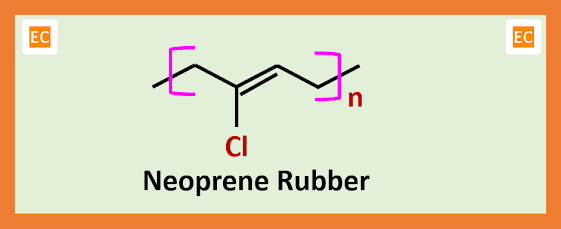
NEOPRENE RUBBER
Neoprene rubber is a type of synthetic rubber which is made by several chloroprene monomer unit. Over a wide range of temperature neoprene maintains its flexibility and exhibits high chemical stability. In market neoprene rubber available in both form, such as solid form and latex form and used in a variety of application.
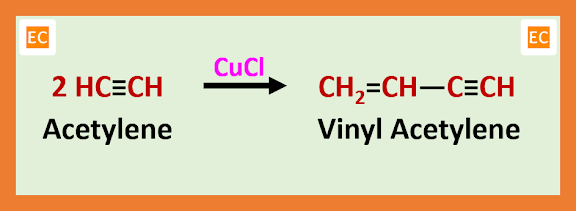
In the manufacture of neoprene rubber, the raw materials required are acetylene (C2H2) and hydrochloric acid (HCl). Acetylene (C2H2) is washed by concentrated sulfuric acid (H2SO4) for purification. In presence of cuprous chloride (CuCl) pure acetylene (C2H2) dimerised to vinyl acetylene (CH2=CH―C≡CH). During this process some amount of divinyl acetylene is also produced, which is separate from vinyl acetylene immediately as an immiscible oil, because this divinyl acetylene violently active compound.
If pure acetylene gas rapidly sweeping through a catalyst solution [this catalyst solution contains concentrated solution of CuCl and NH4Cl at ordinary temperature] then the formation of divinyl acetylene is avoided.
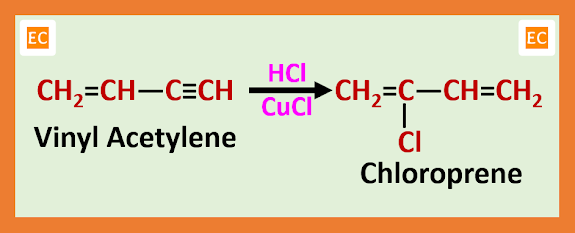
Now the mixture of vinyl acetylene with some amount of divinyl acetylene and some amount of unreacted acetylene is cooled. Due to cooling unreacted acetylene separated as a gas and the mixture of vinyl acetylene and divinyl acetylene are condensed. Now the vinyl acetylene in gas form is passed through a solution of CuCl and concentrated hydrochloric acid (HCl) at 300C. As a result, chloroprene is formed which is the monomeric unit of neoprene rubber.
Now this chloroprene vapour which contain some amount of HCl gas is allowed to cool. As a result, chloroprene condenses to liquid form from vapour form and HCl gas separated which is reused. At ordinary temperature the emulsion polymerization of chloroprene gives neoprene. This emulsion polymerization process is very rapid and this polymerization process follows free radical mechanism pathway.
To increase the plasticity and stabilization of the polymer, tetraethyl thiuram disulphide is added after the polymerization process.
Neoprene can be vulcanized by the following ways----
By heating alone.
By used of ZnO and MgO as a vulcanizing agent.
By used of antimony sulphide.
Properties of Neoprene rubber
Chemically neoprene rubber very closely related to natural rubber, neoprene rubber has higher elongation percentage than natural rubber but tensile strength of neoprene rubber is lower than natural rubber. Neoprene rubber is soluble in polar solvent and it has good weathering resistance. Neoprene rubber is not attacked by alkali below 50% strength and it is resistance to vegetable and mineral oils and also resistance to oxidising agents such as oxygen and ozone and in high temperature.
Application of Neoprene rubber
Neoprene rubber has various uses such as, for making gaskets in oil refining plants, for making various standard engineering material, in conveyor belts, for printing rollers and for making rubber cements.












No comments:
Post a Comment