Synthesis of Albendazole and Mebendazole
Albendazole
Albendazole is a benzimidazole derivative, widely used across the globe for the treatment of neurocysticerosis and echinococcosis and other intestinal nematode infection. Albendazole is found to be quite effective as a single dose treatment for ascariasis. Albendazole is also found in the treatment of infection caused by various worms such as roundworm, whipworm, threadworm and hookworm. A recommended multi dose therapy with albendazole is found to be very effective in the complete eradication of pinworm chlonorchiasis and capillariasis.
Synthesis of Albendazole
Albendazole is prepared by three steps----
Step – 1-----
In this step at first, 3-chloro-6-nitroacetanilide is reacted with propyl mercaptan, as a result 3-propylthio-6-nitroacetanilide is formed. The nitro group present at position 6 in 3-propylthio-6-nitroacetanilide is reduced by hydrogen in presence of palladium on carbon (Pd-C) as a catalyst. As a result, 4-(propylthio)-o-phenylenediamine is formed.
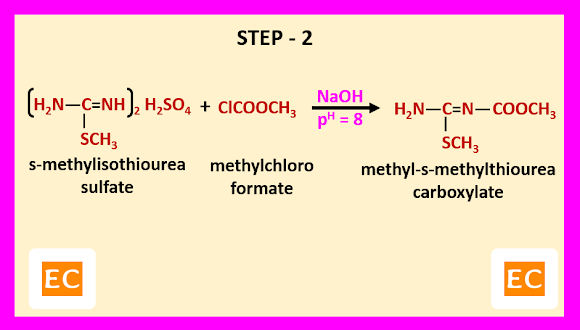
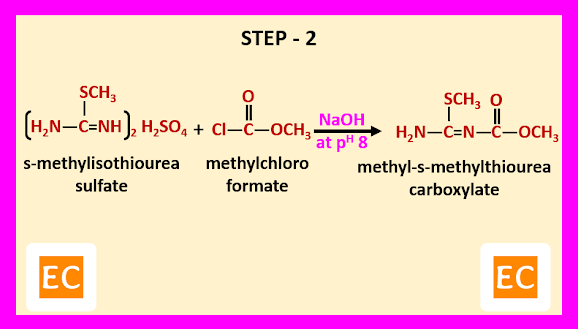
Step – 2-----
In this step, s-methylisothiourea sulfate is reacted with methylchloro formate in presence of NaOH at pH 8. As a result, methyl-s-methylthiourea carboxylate is formed.
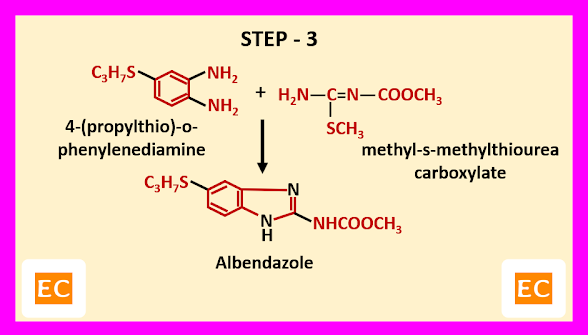
Step – 3------
In the final step, 4-(propylthio)-o-phenylenediamine formed in step–1 and methyl-s-methylthiourea carboxylate formed in step–2, are reacted. As a result, albendazole is formed.
Mebendazole
Mebendazole is also a benzimidazole derivative, widely used across the globe for the treatment of infection caused by hookworm, pinworm, roundworm, whipworm, guinea worm. Mebendazole is used for the treatment of infection caused by Trichuris trichiura, Enterobius vermicularis. Mebendazole is also used as an alternative medicine for Visceral Larva Migrans. Mebendazole is found very effective against nematodes and cestodes.
Synthesis of Mebendazole
Mebendazole is prepared by three steps---
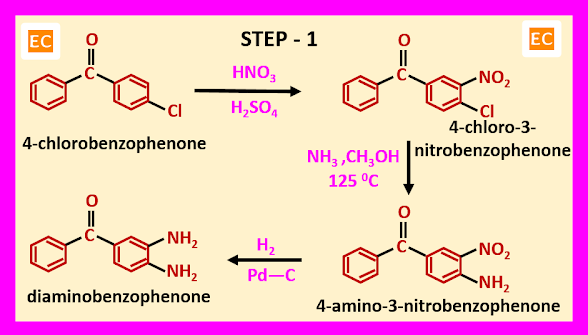
Step – 1-----
In this step at first, 4-chlorobenzophenone reacted with mixed acid (HNO3+H2SO4), as a result 4-chloro-3-nitrobenzophenone is formed. Now 4-chloro-3-nitrobenzophenone reacted with ammonia in presence of methanol at 1250C. As a result, 4-amino-3-nitrobenzophenone is produced. The nitro group present at position 3 in 4-amino-3-nitrobenzophenone is reduced by hydrogen in presence of palladium on carbon (Pd-C) as a catalyst. As a result, diaminobenzophenone is formed.
Step – 2-----
In this step, s-methylisothiourea sulfate reacted with methylchloro formate in presence of NaOH at pH 8. As a result, methyl-s-methylthiourea carboxylate is produced.
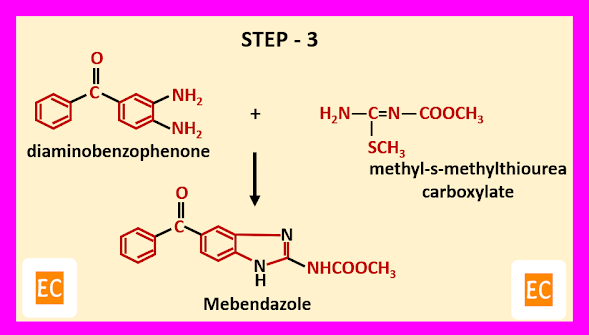
Step – 3-----
In the final step, diaminobenzophenone formed in step-1 and methyl-s-methylthiourea carboxylate formed in step-2 are reacted. As a result, mebendazole is formed.












No comments:
Post a Comment