EFFECT OF CATALYST ON SUBSTITUTION REACTION
Catalytic effect on SN1 reaction---
The rate of SN1 reaction
increases by the addition of Lewis acids such as AlX3, Ag+
ion and the Bronsted acids. The more acidic the nucleophilic solvent, the
faster is the rate of SN1 reaction. Ag+ or Al3+
has a stronger affinity for X- than has a solvent molecule. The
formation of AgX or AlX4- accelerates the dissociation of
X-, thus increases the SN1rate. This is an
example of electrophilic catalysis. The effectiveness of the H bonding, a
factor that accelerates the dissociation of X-, increases with the
acidity of the H of H―A, the Bronsted acid.
Catalytic effect on SN2 reaction---
Crown ethers and phase transfer catalyst accelerate
the rate of SN2 reaction.
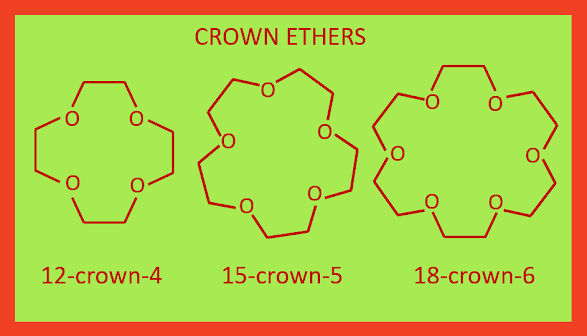
Crown Ethers
The crown ethers contains at least four oxygen (O) atoms, they are the heterocyclic poly ether. The naming of crown ethers is done by the following way x-crown-y,
where ‘x’ represents the total number of atoms in the ring, and ‘y’ represents
the number of oxygen atoms (O). The structure of crown ethers are:
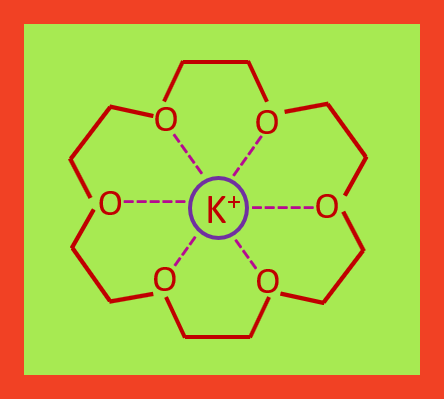
Crown ethers strongly complex with metallic
cation in the cavity of the ring by forming ion-dipole bonds. 18-crown-6
strongly complexes and traps K+ as shown in the figure.
18-crown-6 traps potassium cation
There are a host-guest relationship between the crown ether and
the ion that is transports. There host is crown ether, and guest is the coordinated cation. Similarly, 12-crown-4
forms complex with Li+ and 15-crown-5 forms complex with Na+
ion.
Ion pairing diminishes the reactivity of the
anion which is intended to act as a nucleophile in substitution reaction. By
complexing the cation, the crown ethers leave a ‘bare’ anion with a greatly
enhanced reactivity. Since the nucleophile comes after the rate determining
step in an SN1 reaction, enhancement of nucleophilic
character of an anion does not influence the rate of an SN1
reaction, but it increases the rate of SN2 reaction. For
example, the rate of the reaction---
CH3―CH2―Br + KF -------> CH3―CH2―F
Increases several times by the addition of 18-crown-6.
Phase Transfer Catalyst
A difficulty that occasionally arises when
carrying out nucleophilic substitution reaction is that the reactants do not
mix. For a reaction to take place the reacting molecule is usually insoluble in
water and other polar solvents, while the nucleophile is often an anion which
is soluble in water, but the nucleophile is insoluble in the substrate or other organic solvents. To overcome this problem is to use a solvent that will dissolve both
species. A dipolar aprotic solvent may serve this purpose. Another way, which
is used may often is phase transfer catalysts.
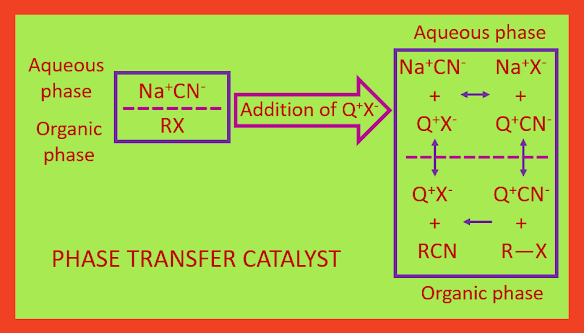
An example of phase transfer catalyst (Q+X-)
is usually a quaternary ammonium halide (R4N+X-),
such as tetra butyl ammonium chloride (Bu4N+Cl-),
benzyl triethyl ammonium chloride [Ph-CH2-N+ (CH2CH3)3Cl-].
These are soluble in organic phase because of the four hydrocarbons
substituents on nitrogen as well as these are soluble in aqueous phase due to
the ionic in nature. The transfer of the
nucleophile (for example CN-) as an ion pair (Q+CN-)
into the organic phase, is causes by the phase transfer catalyst. The reaction between the nucleophile of the ion pair
(CN-) and the organic substrate RX, occur in the organic phase. The cation (Q+)
then migrates back into the aqueous phase to complete the cycle. Until all of the nucleophile or the organic substrate has reacted, the process goes continues.
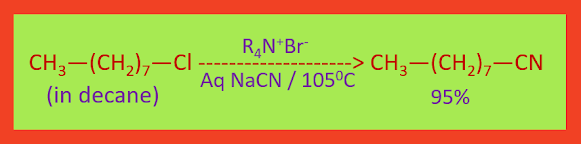
An example of a nucleophilic substitution
reaction carried out with phase transfer catalysis is the reaction of
1-chlorooctane (in decane) and sodium cyanide (in water). The reaction (at 1050C)
is complete in less than 2 hours and gives a 95% yield of the substitution
product.
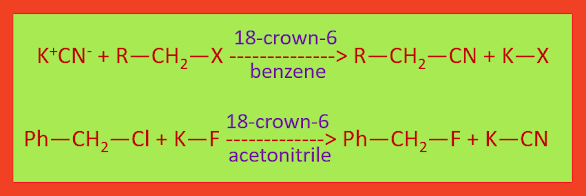
Another example of phase transfer catalyst is crown ethers. They render many salt soluble in non-polar solvents. Salts such as KF, KCN and CH3COOK for example can be transferred into aprotic solvents by using 18-crown-6. A nucleophilic substitution reaction carry out by the relatively unsolvated anions of these salts on an organic substrate in the organic phase.











No comments:
Post a Comment