INTERHALOGEN COMPOUNDS
Halogen atoms have different electronegativity. Therefore, they combine with one another to form a class of compounds known as interhalogen compounds. These may be regarded as the halides of the more electronegative halogens. Since F is the most electronegative of the halogens, it can not form any interhalogen compounds. While the most electropositive iodine has the maximum tendency to form interhalogen compounds.
These compounds can be classified into four groups.
AX type: ClF (g), BrF (g), BrCl (g), ICl (l), IBr (s), IF (unstable)
AX3 type: ClF3 (g), BrF3 (l), ICl3 (s), IF3 (unstable)
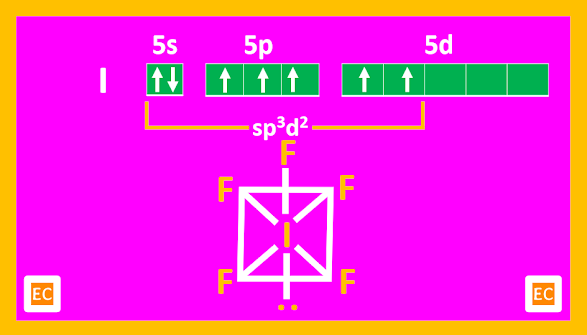
AX5 type: ClF5 (g), BrF5 (l), IF5 (l)
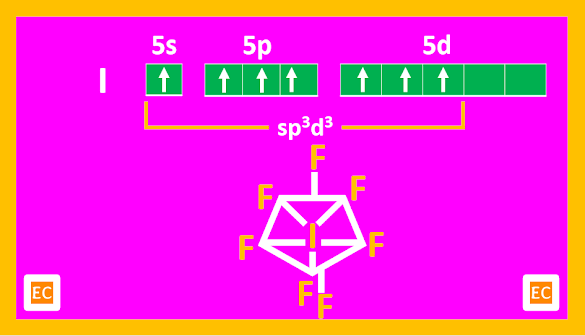
AX7 type: IF7 (g)
[Where A = central halogen atom, X = other halogen atom]
Interhalogens are generally prepared by the direct combination of halogens. These are never than two different halogens in a molecule. Interhalogens are essentially covalent because of small difference in electronegativities between the two halogen atoms. The melting point and the boiling point of the interhalogen compound increases as the difference in electronegativity of two halogens increases.
In general, reactivity of interhalogens are more than halogens (except F2). This is because A―X bond in interhalogen is weaker than the X―X bond in the halogens.
The central halogen atom A has to expand its octet in order to accommodate more than one X group. F is most electronegative and it is devoid of d orbitals. Therefore, it can not serve as the central atom. But F serves strongly as the constituent atom and helps the central atom to attain a high coordination number. Its high electronegativity helps to reduce accumulation of excessive charge density on the central atom.
In interhalogens the number of X atoms is always odd. This is due to the fact that the valence shell of A has or produce odd number of electrons through unpairing.
The melting and boiling points of interhalogens increases regularly with the increase of their molecular weights. This is a proof of their covalent character.
Hydrolysis of an interhalogen produces halide and oxohalide ions. The smaller and the more electronegative halogen forms the halide, while the other forms the oxohalide. The larger and more electropositive halogen serves as the central atom.
The interhalogens are all potential non-aqueous ionizing solvents. This property arises due to their---
1.Convenient liquid range.
2.Good fluorinating behaviour.
3.Considerable self ionisation.
BrF3 is more widely used as a solvent than the others.
2 BrF3 <====> [BrF2] + + [BrF4]-
ICl, IBr, ICl3, IF5 can also be used as non-aqueous ionizing solvents. They have appreciable electrical conductivity in their molten state and in solutions.
2 ICl <====> I+ + [ICl2]-
2 ICl3 <====> [ICl2] + + [ICl4]-
2 IF5 <====> [IF4] + + [IF6]-
2 ClF5 <====> [ClF4] + + [ClF6]-
2 ClF3 <====> [ClF2] + + [ClF4]-
The interhalogens are good fluorinating agents. They are also good oxidants. They fluorinate many metal oxides, metal halides, metals and nonmetals. Interhalogen compounds are reactive. The order of reactivity is---
ClF3>BrF3>IF7>ClF>IF5>BrF>IF3>IF
INDIVIDUAL CATEGORY
AX type compounds
All six compounds such as ClF, BrF, IF, BrCl, ICl, and IBr are known.
General properties
1.These are essentially covalent.
2.The melting points and boiling points of these interhalogens are intermediate between two free halogens which made the interhalogen.
3.Their thermal stability falls in the order—
IF>BrF>ClF>ICl>IBr>BrCl
Corresponding to the electronegativity difference between the two atoms. The thermal stability of the molecule increases when the polarity increases.
4.Lewis acid strength decreases in the order---
ICl>BrCl>IBr
5.When molten ICl is electrolyzed, a mixture of I2 and Cl2 is liberated at anode and only I2 is liberated at cathode. This mode of electrolysis suggests that ICl ionizes as---
2 ICl <====> I+ + ICl2-
At cathode:
2 I+ + 2 e- ------> I2
At anode:
2 ICl2- - 2 e ------> I2 + 2 Cl2
6.With alkali halides they form ionic poly halides.
NaBr + ICl ----> Na+[BrICl]-
KCl + ICl ----> K+[ICl2]-
7.AlCl3, SiCl4, SnCl4, SbCl5 produce I+ ion in liquid ICl and hence are acidic.
AlCl3 + ICl -----> I+ + AlCl4-
SiCl4 + ICl ----> I+ + SiCl5-
SbCl5 + ICl ----> I+ + SbCl6-
8.ClF is a good fluonating agent. It can fluorinate metals and non-metals.
6 ClF + 2 Al -----> 2 AlCl3 + 3 F2
6 ClF + S ------> SF6 + 3 Cl2
It can simultaneously chlorinate and fluorinate a compound.
ClF + SF4 ------> SF5Cl
ClF + SO2 -----> ClSO2F
ClF + CO -----> COFCl
Structure
The AX type of interhalogen has linear structure.
AX3 type of compounds
Known compounds are ClF3, BrF3, (ICl3)2, IF3. ClF3 and BrF3 are well known. These are covalent. ClF3 is the most reactive but BrF3 is most useful as a fluorinating agent since it is a liquid and is not too violent in its action.
All the trifluorides are powerful oxidants and fluorinating agents. The oxidising power increases in the order---
IF3<BrF3<ClF3
Their thermal stabilities are in the order---
BrF3>ClF3>ICl3>IF3
The order of their reactivity---
ClF3>BrF3>IF3
These undergo self ionisation considerably; these have high electrical conductivity.
2 ClF3 <=====> [ClF2] + + [ClF4]-
2 BrF3 <====> [BrF2] + + [BrF4]-
2 ICl3 <=====> [ICl2] + + [ICl4]-
Fluorinating behaviour:
ClF3 + BF3 -----> [ClF2] + [BF4]-
ClF3 + SbF5 -----> [ClF2] + [SbF6]-
ClF3 + PtF5 -----> [ClF2] + [PtF6]-
4BrF3 + 3SiO2 ----> 3SiF4 +2Br2 +3O2
Structure
The X-ray structure of crystalline ClF3 shows that the molecule is T-shaped, with bond angle of 87040'.
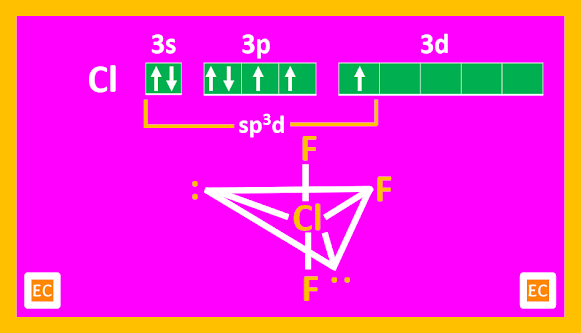 |
| Structure of ClF3 |
The distortion from 900 is due to repulsion between the lone pairs. The structure is trigonal bipyramid arising out of the sp3d hybridization of Cl.
The structure of BrF3 is also T-shaped. ICl3 in the solid state is a dimeric planar molecule, (ICl3)2. Here two T-shaped ICl3 molecules join together forming the dimer.
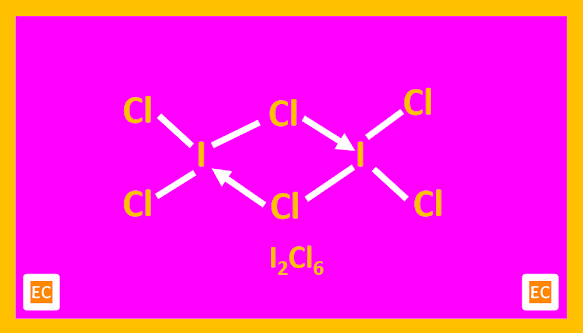 |
| Structure of I2Cl6 |
AX5 type of molecules
Three interhalogen compounds such as ClF5, BrF5, and IF5 are known.
The order of reactivity is---
BrF5>ClF5>IF5
They fluorinate compounds, react violently with water.
ClF5 + 2H2O = FClO2 + 4HF
BrF5 + AsF5 = [BrF4] + [AsF6]-
BrF5 + CsF = Cs+[BrF6]-
IF5 + KF = K+ [IF6]-
IF5 + SbF5 = [IF4] + [SbF6]-
Liquid IF5 self ionizes and therefore conducts electricity.
2 IF5 <====> [IF4] + [IF6]-
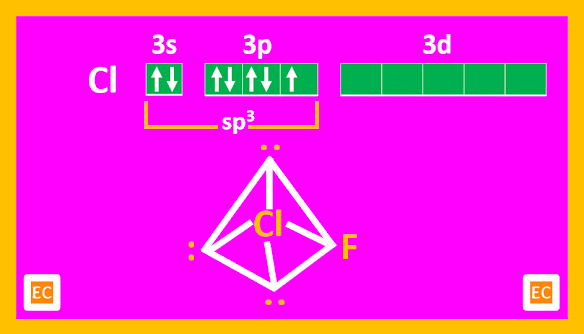
Structure
AX5 compounds all have square pyramidal structures (octahedral with one position occupied by a lone pair).
AX7 type of compounds
Only known compound is IF7. It is a gas at ordinary temperature and is highly reactive. It is a violent fluorinating agent.
IF7 + 6H2O <====> H5IO6 + 7HF
IF7 + CsF <====> Cs+ [IF8]-
IF7 + SbF5 <====> [IF6] + [SbF6]-
Structure
The structure of IF7 is unusual. It is pentagonal bipyramidal in shape.











No comments:
Post a Comment