Fullerenes
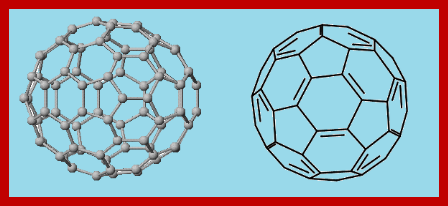
Fullerene is the allotropes of carbon and commonly refers to a molecule with 60 carbon atoms, C60, and with an icosahedral symmetry. Now a days there are several such hollow closed-cage (polyhedral) cluster molecules, Cn (n only even in the range 30-600), and all of them have structures based on polyhedra formed by fusing pentagons and hexagons. Larger molecular weight fullerenes are C70, C76, C78, C80 which possess different geometric structure, such as C70 has a rugby ball-shaped symmetry. The structure of fullerenes very much comparable to geodesic domes used in architecture built by R. Buckminster Fuller. The most widely used fullerene C60, was called buckminster fullerene or buckyball.
Preparation of Fullerenes
Synthesis of fullerenes is done by arc discharge between graphite electrodes in approximately 200 Torr of He gas. The formation of fullerenes occurs due to evaporation of carbon, because of heat generated at the contact point between the electrodes. This discharge produces a carbon soot that can contain upto 15% fullerenes, C60 (13%) and C70 (2%). The fullerenes are separated according to their mass, by use of liquid chromatography and using a solvent such as a toluene.
Properties of Fullerenes
The colour of thin films of C60, are mustard-coloured, but in bulk it appears dark brown. Where as the colour of thin films of C70, are red-brown coloured, but in bulk it appears grey-black. The colour of the solutions of C60, is magenta colour, where as the colour of the solutions of C70, are dull red. Fullerenes are dissolved slowly in organic solvents, indicating close packing in the crystal and have high melting points. The C-13 nmr spectrum of C60, shows a single peak (142.68 pm), suggesting uniform environment for all 60 carbon atoms.
Structure and Bonding of Fullerene
In fullerene molecule, each carbon may be supposed to sp2 hybridized, and forming three σ
bonds to other three carbon atoms. Therefore, the remaining electron at each carbon is delocalized into a system of molecular orbitals that imparts some aromatic character to the whole molecule. One mechanism suggested that, the addition of carbon atoms occur, first in pentagonal and hexagonal patterns which gradually curl under appropriate conditions to form the cage-like structure. To close into a spheroid, a fullerene must have exactly 12 five membered faces, but the number of six membered faces can vary widely (1/2 n - 10).The number of five membered or pentagonal faces are 12 in C60, whereas number of six membered or hexagonal faces are [1/2 (60) - 10] = 20. In case of C70, the number of five membered or pentagonal faces are 12, and number of six membered or hexagonal faces are [1/2 (70) - 10] = 25. In the case of C60, each carbon atom is at the junction of two six membered rings and one five membered ring. Each six membered rings containing three double bonds. Thus C60 has the structure of a truncated icosahedron. Fullerenes have two different types of C―C bonds:
(a) Those at an edge shared between two fused hexagons, that is ( 6 : 6 rings), the bond length is 139.1 ± 1.8 pm. This is also the distance between pentagons.
(b) Those at an edge between a pentagon and a hexagon or the bonds within a given pentagon are somewhat longer, the bond length is 145.5 ± 1.2 pm.
The unit cell of solid C60 consists of a face centered cubic array of C60 molecule in close packing. The molecules can rotate freely at their lattice sites by the thermal energy available at room temperature and can be considered to be spherical.











No comments:
Post a Comment