Carbocation
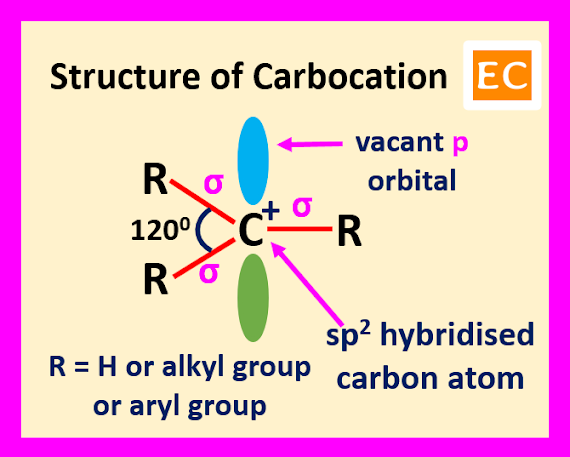
When an organic species containing a carbon atom and that carbon atom bearing only three electron pairs, means six electrons is called a carbocation. Carbocation containing a positive charge and the carbon atom of the carbocation is sp2 hybridized. The carbon atom of the carbocation uses its three hybrid orbitals for forming three sigma bonds with three substituents and the remaining p orbital remains vacant. The structure of carbocation is flat structure, having all the three covalent bonds in one plane. The bond angles between these covalent bonds are 1200.
Formation of Carbocation
Carbocation can be formed in a number of ways. Some of these reactions by which carbocations are generated are summarized below---
(1) Carbocation is formed by the solvolysis of C―X bond (where X = halogen such as F, Cl, Br, I or OBs etc) ---
R―X -----> R+ + X-
(2) When alkyl halide (R―X) reacts with superacids such as SbF5, HSO3F etc, carbocation is formed --
R―F + SbF5 -----> R+ + SbF6-
(3) When acetyl chloride (R―CO―Cl) reacts with aluminium chloride (AlCl3) carbocation is generated---
R―CO―Cl + AlCl3 ----> R―C+=O + AlCl4-
(4) When nitrous acid (HNO2) reacts with amine (R―NH2), carbocation is produced--
R―NH2 + HNO2 ------> R+ + N2 + H2O + OH-
(5) Protonation of alcohol (R―OH) by acid, followed by dehydration produced carbocation-
R―OH + H+ -----> R―OH2+
R―OH2+ -----> R+ + H2O
(6) When carbonyl compound reacts with H+, carbocation is formed----
R―CO―R + H+ -----> R2C+―OH
(7) When alkene reacts with H+, carbocation is formed----
R―CH=CH2 + H+ -----> R―CH+―CH3
(8) When allyl halide reacts with Ag+, allyl carbocation is formed----
CH2=CH-CH2-Br + Ag+ ---> CH2=CH-CH2+ + AgBr
(9) When benzyl halide reacts with Ag+, benzyl carbocation is formed-----
Ph―CH2―Br + Ag+ ----> Ph―CH2+ + AgBr
Classification of Carbocation
Carbocations are classified into three categories----
Primary Carbocation
When a positively charged carbon ion linked with another carbon atom, then this carbocation is called primary carbocation.
CH3―CH2+
Secondary Carbocation
When a positively charged carbon ion linked with other two carbon atoms, then this carbocation is called secondary carbocation.
CH3―CH+―CH3
Tertiary Carbocation
When a positively charged carbon ion linked with other three carbon atoms, then this carbocation is called tertiary carbocation.
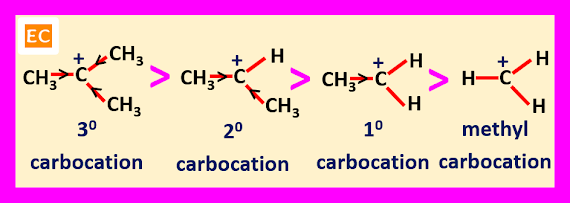
Stability of Carbocation
The relative stabilities of the alkyl substituted carbocations are----
R3C+ > R2CH+ > RCH2+ > CH3+
The stabilities of the alkyl substituted carbocations can be explained by various factors, which are---
Inductive Effect
The alkyl groups have +I effect, so they tend to release electrons and partially compensate for the electron deficiency of the positively charged carbon. When the number of alkyl groups attached to the positively charged carbon atom increases, electrons release by this alkyl groups increases and partially compensate for the electron deficiency of the positively charged carbon also increases. So, the stability of tertiary carbocation (containing three alkyl groups) is greater than secondary carbocation (containing two alkyl groups). The stability of secondary carbocation is greater than primary carbocation (containing only one alkyl group). The stability of primary carbocation is greater than methyl carbocation (containing no alkyl group).
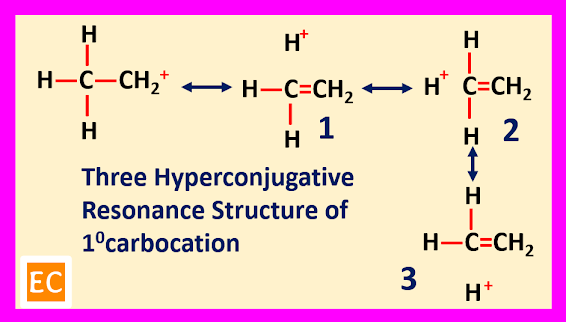
Hyperconjugation
The sigma (σ) electrons of an alpha (α) C―H bond can be delocalized into the unfilled ‘p’ orbital of the positively charged carbon atom. Thus, spreading the charge over all such bonds. So, several hyper conjugative resonance structure can be drawn for alkyl substituted carbocation and each of these resonance structure containing same number of covalent bonds as the first structure.
For primary carbocation three structure are drawn, for secondary carbocation six structure are drawn and for tertiary carbocation nine structure are drawn. With increase the number of hyper conjugative resonance structure, the stabilities of the carbocation increases.
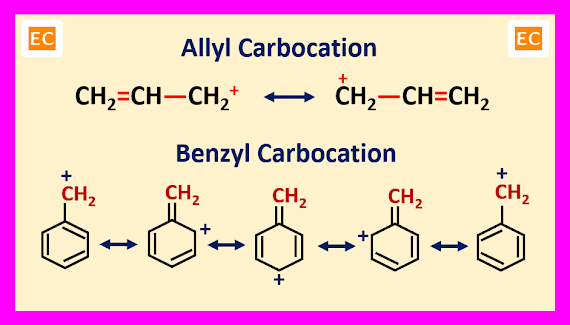
Mesomeric Effect or Resonance Effect
The stabilities of conjugated carbocations can be explained by mesomeric effect or resonance effect. Resonance enhances the stability of a carbocation by delocalization of its charge in conjugated system like allyl or benzyl carbocation. More number of resonating structures of a carbocation, more will be its stability. The order of stability of allyl and benzyl carbocations are----
CH2=CH-CH2+<Ph-CH2+<(Ph)2CH+<(Ph)3C+
Number of resonating structures of allyl carbocation is 2, whereas number of resonating structures of Ph―CH2+ is 5, (Ph)2CH+ is 9 and (Ph)3C+ is 13.
Steric Effect
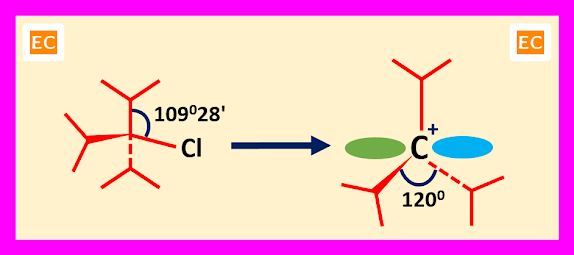
When carbocations derived from highly substituted substrate, steric effects play an important role in the formation and stability of carbocations. Here steric relief is the key factor for the formation and stability of carbocations. Tri-isopropyl chloride is a highly substituted substrate. In tri-isopropyl chloride three bulky isopropyl groups are pushed together due to sp3 angle of 109028'. So due to this pushing together, there arises a strain called B-strain. When the substrate tri-isopropyl chloride ionizes, the angle expands from 109028' to 1200. So, a relief in strain arises due to this angle expands and space between alkyl groups (isopropyl groups) increases.












No comments:
Post a Comment