PREPARATION
OF ALKANES
Alkanes can
be prepared by various ways. General methods of preparation of alkanes are--
(1) From
Unsaturated Hydrocarbons
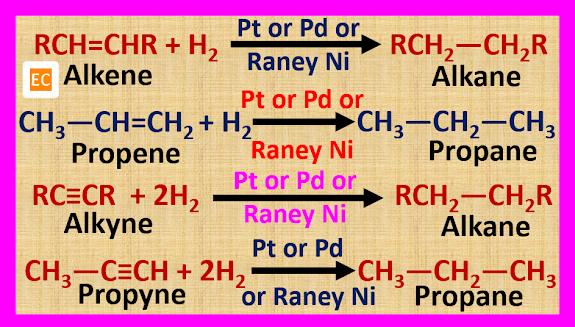
Alkanes are
prepared by the catalytic reduction of unsaturated hydrocarbons such as alkene
or alkyne in the presence of a suitable catalyst such as Ni, Pt, Pd, Raney Ni
etc. Here hydrogen is added to an unsaturated hydrocarbon (alkene or alkyne) in
the presence of a catalyst. This process is known as hydrogenation.
The catalytic
hydrogenation reaction takes place at normal temperature and pressure if we use
Raney Ni, Pt or Pd as a catalyst.
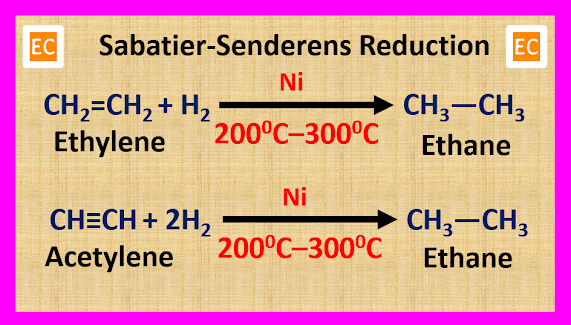
But if we use
Ni as a catalyst, then the catalytic hydrogenation reaction takes place at 200 0C – 300 0C. The catalytic hydrogenation reaction in presence of a Ni catalyst
at 200 0C – 300 0C is known as Sabatier-Senderens reduction.
(2) From
Alkyl Halides
Alkanes can
be prepared from alkyl halides in different ways---
(A) Through
Grignard Reagent
When alkyl
halide heated with magnesium powder in ether solution, alkyl magnesium halide
(R―Mg―X)
is produced. This alkyl magnesium halide (R―Mg―X)
is known as Grignard reagent.
R―X
+ Mg → R―Mg―X
C2H5―I
+ Mg → C2H5―Mg―I
When Grignard
reagent treated with water or dilute acid, alkane is produced.
R―Mg―X
+ H2O → RH + Mg(OH)X
C2H5―Mg―I
+ H2O → C2H6 + Mg(OH)I
(B) By Wurtz
Reaction
In dry ether
solution at normal temperature the reaction of two molecules of alkyl halide
(preferably the bromide or iodide) with two molecules of pure and dry metallic sodium produce alkane.
R1―X
+ 2Na + R2―X → R1―R2
+ 2NaX
CH3―Br
+ 2Na + CH3―Br →
CH3―CH3
+ 2NaBr
If we use two
types of alkyl halide, then mixture of alkanes is produced. It is difficult to
separate this mixture of alkanes, because their close boiling point.
CH3―Br
+ 2Na + C2H5―Br →
CH3―CH3 + C2H5―C2H5
+ CH3―C2H5 + 2NaBr
This Wurth
reaction is very useful for the synthesis of symmetrical alkane.
(C) By Corey-House
Synthesis
In dry ether
solution at normal temperature the reaction of alkyl halide with pure and dry metallic
lithium produced alkyl lithium.
RX + 2Li →
RLi + LIX
C2H5I
+ 2Li → C2H5Li + LiI
Reaction of alkyl
lithium (RLi) with cuprous iodide (CuI) produced lithium dialkyl cuprate (R2CuLi).
2RLi + CuI → R2CuLi
+ LiI
2C2H5Li
+ CuI → (C2H5)2CuLi + LiI
Reaction of
lithium dialkyl cuprate (R2CuLi) with another molecule of alkyl
halide produced alkane.
R2CuLi
+ R'X → R―R' +
RCu +LiX
(C2H5)2CuLi
+ CH3I → C2H5―CH3
+ C2H5Cu + LiI
R and R'
may be same or different. R group may be primary, secondary or tertiary but R'
group always primary.
This
Corey-House reaction is very useful for the formation of both symmetrical and
unsymmetrical alkane.
(D) Reduction
of Alkyl Halides
(i) Alkane is
prepared by the reduction of alkyl halide by Zn and HCl.
C2H5Cl
+ Zn + HCl → C2H6 + ZnCl2
(ii) Alkane
is prepared by the reduction of alkyl halide by Zn and NaOH.
Zn + 2NaOH →
Na2ZnO2 + 2[H]
R―X
+ 2[H] → R―H + HX
(iii) When
alkyl halide is reduced by Zn-Cu couple in presence of alcohol alkane is
produced.
Zn →
Zn2+ + 2e
R―X
+ e → R∙ + X-
R∙ +
e →
R:-
R:-
+ C2H5OH → R―H
+ C2H5O-
(iv) When
alkyl halide is reduced by LiAlH4, NaBH4 or Ph3SnH
alkane is produced.
R―X
+ H- → R―H + X-
(v) When
alkyl halide is reduced by H2 in presence of Pd-C or Raney Ni,
alkane is produced.
R―X
+ H2 → R―H + HX
(vi) When
alkyl iodide is reduced by hydroiodic acid (HI) and red phosphorus at 150 0C,
alkane is produced.
C2H5―I
+ HI → C2H6 + I2
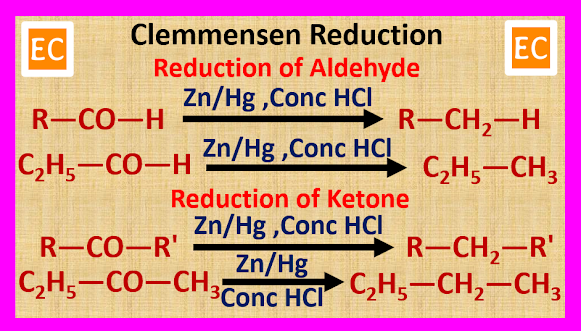
(3) From
Carbonyl Compounds
Alkane can be
prepared from carbonyl compounds such as aldehyde and ketone by Clemensen
reduction process and Wolf-Kishner reduction process.
(A) By
Clemmensen Reduction
When carbonyl
compounds such as aldehydes and ketones are heated with zinc amalgam (Zn/Hg)
and concentrated HCl, carbonyl compounds reduced to form alkane.
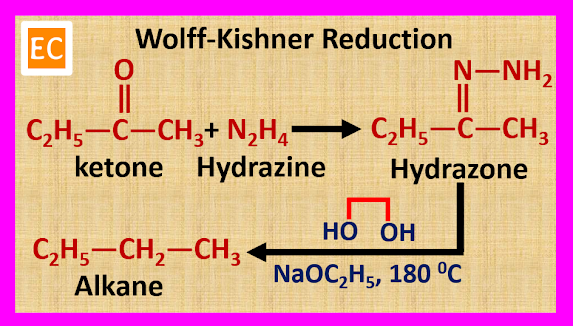
(B) By
Wolff-Kishner Reduction
Carbonyl
compounds such as aldehydes and ketones react with hydrazine (H2N―NH2)
to formed hydrazone. When the resulting hydrazone is heated with sodium
ethoxide (NaOC2H5) and ethylene glycol (HOH2C―CH2OH)
at 180 0C, alkane is produced.
(4) From
Carboxylic Acid
Alkane can be
prepared from carboxylic acid by two methods. One is decarboxylation process
and another is Kolbe’s electrolytic method.
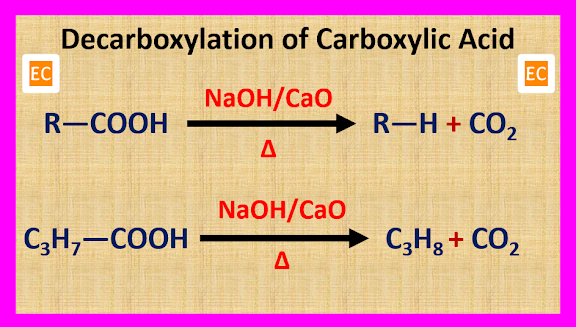
(A) By
Decarboxylation Process
Alkane is
produced when carboxylic acid is heated with soda lime (NaOH + CaO). In this
reaction CO2 is removed, so it is called decarboxylation reaction.
This is a
degradation reaction, because in this process the number of carbon atom in the
produced alkane is one less than that of the carboxylic acid.
(B) Kolbe’s
Electrolytic Method
Electrolysis
of a concentrated and cool aqueous solution of sodium or potassium salts of monocarboxylic
acid using platinum electrodes produced alkane at the anode.
2R―COOK
+ 2H2O → [R―R + 2CO2(anode)] + [H2
+ 2KOH (cathode)]
2CH3COOK
+ 2H2O → [C2H6 + 2CO2(anode)]
+ [H2 + 2KOH (cathode)]
Kolbe’s
method of electrolysis is suitable for the preparation of even number of carbon
atom alkane, but not suitable for the preparation of odd number of carbon atom alkane.
(5) From
Alkyl Borane
Reaction of
alkene with borane produced trialkyl borane.
3R―CH=CH2
+ B2H6 → (R―CH2―CH2)3B
Alkane is produced
by hydrolysis of the resulting trialkyl borane with acid.
(RCH2CH2)3B
+ 3CH3COOH → 3RCH2CH3 + (CH3COO)3B
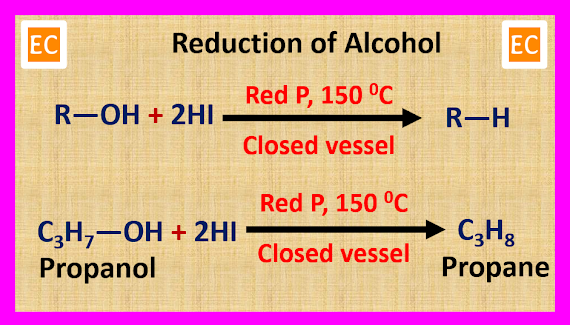
(6) From
Alcohol
When alcohol
is reduced by red phosphorus (P) and hydroiodic acid (HI) in a closed vessel at
150 0C, alkane is produced.