BORAX
Salts of boric acids (H3BO3) are called borates. Borax is an important borate. Borax is a sodium salt of tetraboric acid (H2B4O7). Ten molecules of water, as water of crystallization are present in borax compound. So, borax is sodium tetraborate decahydrate [Na2B4O7.10H2O].
Preparation of Borax
(1) Borax from boric acid (H3BO3) ---
(i) Borax is obtained, when boric acid (H3BO3) is boiled with sodium carbonate (Na2CO3) solution-
4H3BO3 + Na2CO3 = Na2B4O7 + CO2 + 6H2O
(ii) Borax is obtained, when boric acid (H3BO3) is boiled with excess sodium hydroxide (NaOH) solution---
4H3BO3 + 2NaOH + 3H2O = Na2B4O7.10H2O
(2) Borax from colemanite (2CaO, 3B2O3) ---
Borax is obtained, when colemanite (2CaO, 3B2O3) is boiled with sodium carbonate (Na2CO3) solution ---
2CaO, 3B2O3 + 2Na2CO3 = Na2B4O7 + 2NaBO2 + 2CaCO3
At first, precipitate calcium carbonate (CaCO3) is filtered out and then concentrated the remaining filtrate. As a result, crystals of borax are deposited. NaBO2 present in the mother liquor is converted to borax by passing CO2.
4NaBO2 + CO2 = Na2B4O7 + Na2CO3
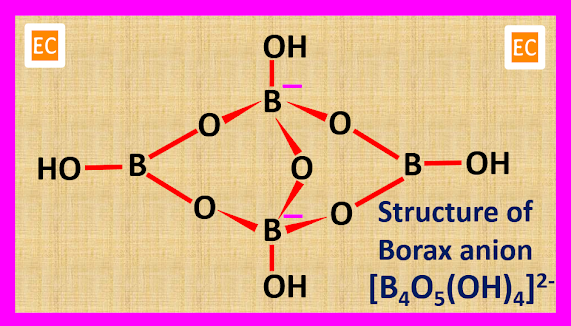
Structure of Borax
The crystal structure study of borax has indicated that, two H2O molecules participate in the formation of borax anion. So, borax is now represented as----
Na2[B4O5(OH)4].8H2O
The anion of borax [B4O5(OH)4]2- is made up of two trigonal planer BO3 units and two tetrahedral BO4 units. The B atom of BO3 unit is sp2 hybridised and each B atom of BO3 unit forms three B―O sigma(σ) bonds. The B atom of BO4 unit is sp3 hybridised. one extra electron is present in each BO4 units and each B atom of BO4 unit forms four B―O sigma (σ) bonds. Four OH groups linked with four B atoms. A bridge bond is formed among two B atoms and one O atom. So, the structure of the borax anion [B4O5(OH)4]2- is as follows---
Properties of Borax
White crystalline solid borax exists in three different crystal structure.
(1) Monoclinic or prismatic borax---
The formula of monoclinic or prismatic borax is Na2B4O7.10H2O, it is the most common form of borax.
(2) Octahedral or jeweller’s borax---
The formula of octahedral or jeweller’s borax is Na2B4O7.5H2O. The octahedral form of borax (Na2B4O7.5H2O) is obtained, when monoclinic form of borax (Na2B4O7.10H2O) is heated above 600C.
(3) Borax glass or anhydrous borax----
The formula of borax glass or anhydrous borax is Na2B4O7. The anhydrous borax (Na2B4O7) is obtained when monoclinic form of borax (Na2B4O7.10H2O) is heated above its melting point.
In water borax gets hydrolysed, and gives a mixture of strong base NaOH and weak acid H3BO3. Since boric acid (H3BO3) is weak so, the aqueous solution of borax is alkaline.
Na2B4O7 + 7H2O <=====> 2NaOH + 4H3BO3
On heating borax first swells losing water molecules. On further heating it gives NaBO2 and B2O3. A glassy bead is formed.
Na2B4O7.10H2O = 2NaBO2 + B2O3 + 10H2O
Borax bead test-----
When this glassy bead (B2O3) is heated strongly with coloured metal salts, specific coloured beads are obtained. This offers a means of identifying certain metal ions like Cu, Co, Cr, Ni, Fe etc.
CuSO4 = CuO + SO3
CuO + B2O3 = Cu(BO2)2
This gives green colour in hot condition and blue colour in cold condition.
When this Cu(BO2)2 is again heated in reducing flame it becomes dull red and opaque.
2Cu(BO2)2 (Cu = +2) + C ----> 2CuBO2 (Cu = +1) + B2O3 + CO
2CuBO2 (Cu = +1) + C ----> 2Cu (Cu = 0) + B2O3 + CO
Reaction of borax with conc HCl or H2SO4 forms boric acid---
Na2B4O7 + 2HCl + 5H2O = 2NaCl + 4H3BO3
Na2B4O7 + H2SO4 + 5H2O = Na2SO4 + 4H3BO3
When borax is heated with ammonium chloride, boron nitride is formed---
Na2 [B4O5(OH)4] + 2NH4Cl ----> 2NaCl + 2BN + B2O3 + 6H2O
Uses of Borax
Borax is used in the manufactured of enamels, washing powders, various types of glasses such as optical and hard glasses. Borax is also used in making antiseptic.











No comments:
Post a Comment