Ozone Layer
The ozone layer is mainly found in the lower region of the stratosphere in the atmosphere, the ozone layer lying between 15 km to 35 km from the earth's surface. Ozone is an allotrope of oxygen. Ozone is a pale blue colour gas with a distinctively pungent smell. The ozone layer is very useful for human beings, animals and plants living on the earth. The radiations emitted by the sun and reaching on the earth consists of ultra-violet radiations. These radiations are very harmful to the living organism. These radiations are of short wavelength and hence penetrate deep into our body and cause skin cancer. These radiations also damage crop plant. Ozone layer present in the stratosphere region of the atmosphere absorbs harmful ultra-violet radiations coming from the sun and thus prevent these radiations from reaching earth. Thus we are saved from the harmful effects cause by the ultra-violet radiations.
Formation of ozone layer
Ozone is an essential protective agent in the stratosphere. It is formed by photochemical dissociation of oxygen:
O2
+ hv -----> O• + •O*
(This requires light of λ<242 nm, in the far UV.)
The activated oxygen atoms, O*, react with molecular oxygen to form ozone:
•O* + O2 + M ------> O3
+ M
The ozone formed in this way absorbs ultraviolet radiation with λ < 340 nm, regenerating molecular oxygen:
O3 + hv -----> O2
+ O•
followed by
O• + O3 ------> 2O2
This mechanism filters out much of the sun's ultraviolet radiation, protecting plant and animal life on the surface of the earth from other damaging photochemical reactions. Causes of the ozone hole formation
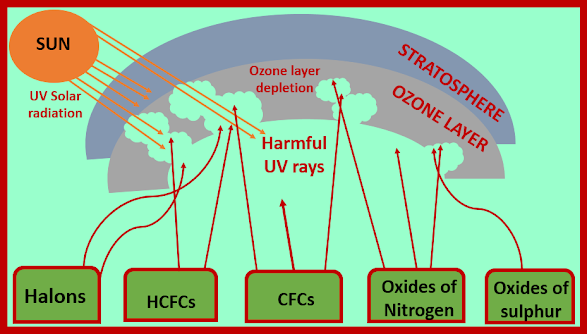
The ozone hole formation occurs due to the compounds added to the atmosphere by humans. Chlorofluorocarbons, especially CF2Cl2
and CCl3F, known as CFC 12 and 11 respectively are the most important type of these compounds. These compounds widely used as refrigerants, blowing agents for the manufacture of plastic foams. The destruction of ozone by these compounds is caused, paradoxically, by their extreme stability and lack of reaction under ordinary conditions. Due to their stability, they remain in the atmosphere indefinitely and finally diffuse to the stratosphere. The intense high energy ultraviolet radiation in the stratosphere causes dissociation and forms chlorine atoms, which then undergo a series of reactions that destroy ozone:
CCl2F2 + hv -------> Cl• +
•CClF2
(This requires ultraviolet radiation with λ = 200 nm)
Cl• + O3 ------> ClO• + O2
ClO• + O• -------> Cl• + O2
(These two reactions remove O3 and oxygen atoms without reducing the number of chlorine atoms, Cl•.)
Other compounds, such as NO and NO2, also contribute to the chain of events:
NO• + ClO• -------> •Cl + NO2•
NO2• + O3 -------> NO3• + O2
NO• + O3 ------> NO2• + O2
NO2• + NO3• + M -------> N2O5
+ M
The chains are terminated by reactions such as
•Cl + CH4 -------> HCl + •CH3
•Cl + H2 ------> HCl + H•
That are followed by combinations of the new radicals to form
stable molecules such as CH4, H2, C2H6,
and by a reaction that ties up the chlorine:
ClO• + NO2• + M --------> ClONO2 + M
During the winter, a combination of air flow pattern and low temperature create stratospheric clouds of ice particles. The surface of these particles is an ideal location for reaction of NO2, OCl, and O3. These clouds contain
nitric acid hydrate, formed by
N2O5 + H2O -------> 2 HNO3
ClONO2 + H2O ------> HOCl + HNO3
These reactions, plus
HCl + HOCl ------> Cl2 + H2O
HCl + ClONO2 ------> Cl2 + HNO3
remove chlorine from the air and generate Cl2 on the surface of the ice crystals. In the spring, increased sunlight splits these molecules into chlorine atoms, and the decomposition of ozone proceeds at a much higher rate. The reaction with NO2 that would remove ClO from the air is prevented because the NO2 is mostly tied up as HNO3 in the ice. The polar vortex prevents mixing with air containing a higher concentration of ozone, and the result is a reduced concentration of ozone over the Antartic. As the air warms in the summer, the circulation changes, the clouds dissipate, and the level of ozone returns to a more nearly normal level.
Volcanic activity that injects SO2 into the atmosphere also has an effect that depends on temperature and on the height of the SO2 injection. The SO2 reacts with air to form SO3, which then reacts with water to form sulfuric acid aerosols. These volcanic aerosols particularly at cold polar temperatures, reduced the nitrogen oxide concentration of the air and active chlorine species that destroy ozone. Because these aerosols are stable at warmer temperatures (200 K) than the natural stratospheric clouds, and because they can exist at lower altitudes, they can have significant effects. Until the level of chlorine is reduced to preindustrial levels, low temperatures and volcanic activity are likely to create Arctic ozone holes each spring as a result of reactions during the winter.
Within two years of the discovery of the ozone hole, the international community had accepted this as evidence of a global problem, and the Montreal Protocol on Substances that Deplete the ozone layer was signed. it set a schedule for decreasing use and production of CFCs and eventually for their complete ban.
 |
| Ozone Layer Depletion |












No comments:
Post a Comment