Vulcanization of Rubber
The wide application of rubber is due to its peculiar property, called elasticity and that is why the rubber is said to be elastoplastic or elastomer. However crude rubber is of little use as such, because it has very undesirable properties. It possesses elasticity only over a limited range of temperature and does not regain its original shape after being stretched. It becomes soften strictly on heating and brittle on cooling. It has low tensile strength and poor resistance to abrasion. By vulcanization, all these defects are removed, and a material of high tensile strength, sufficient toughness, high elasticity, and a non-sensitivity to a wide change of temperature is obtained. Vulcanization is carried out by heating crude rubber in presence of sulfur or dipping it in a solution of S2Cl2 in CS2. Vulcanization depends upon the amount of sulfur used (by increasing the amount of sulfur the rubber can be hardened), temperature, and duration of heating.
Vulcanization is a progressed reaction and therefore it is only allowed to a definite stage. The time of vulcanization and temperature is reduced by adding accelerators and activators. When the amount of sulfur is increased, the rubber is hardening and by increasing the percentage of sulfur to 40-45%, a non-elastic substance, called ebonite is obtained.
Vulcanization is a free radical initiated chain reaction. The free radical is furnished by the accelerator by removing the hydrogen ion from the isoprene monomer forming an active center. The rubber on vulcanization undergoes a great reduction in plasticity, whereas elasticity is largely maintained. Actually, in the vulcanization process, sulfur adds at the unsaturated bonds forming bridges and cross-linking the linear molecules into a practically infinite three-dimensional structure. After the molecular chains are cross-linked, the rubber loses plasticity and acquires elastic properties.
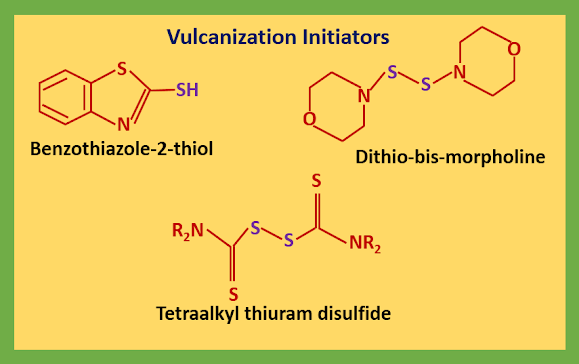
In the vulcanization process, the raw rubber is heated with sulfur, and cross-linking of the polyisoprene chains with short chains of sulfur atoms give it extra strength without destroying the elasticity. Nowadays, a vulcanizing initiator, usually a thiol or a simple disulfide, is added as well.
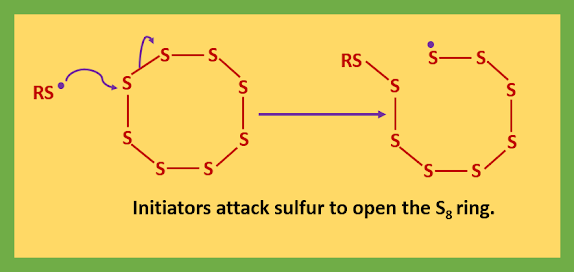
The thiols give sulfur radicals and the disulfides cleave easily as the S-S bond is weak. The initiator (RS˙) either attack the rubber directly or attack sulfur to open the S8 ring.
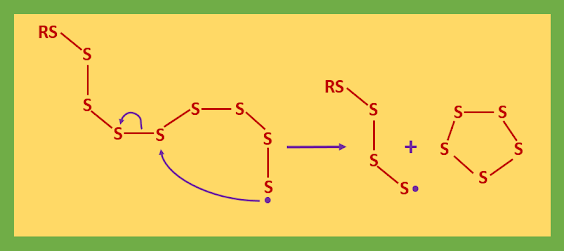
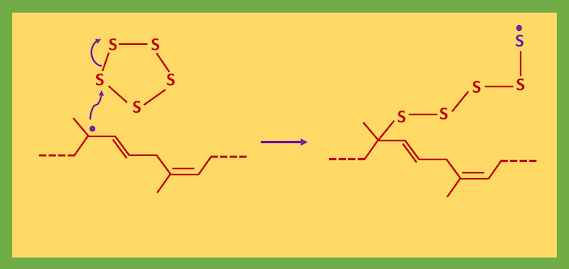
As a result of this attack, the released sulfur radical can bite back on to the sulfur chain and form a closed ring of 5-7 sulfur atoms and forms a short chain of sulfur atoms attached to the initiator and terminating in a sulfur radical.
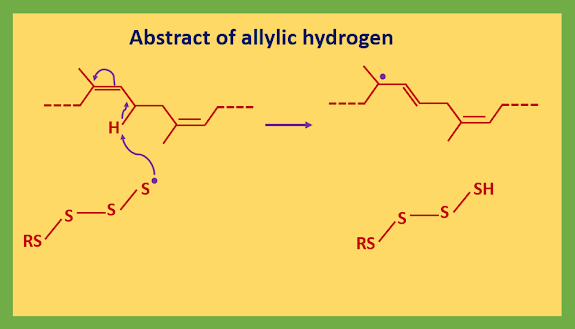
Now the process for the attack on rubber can start. The vulcanized rubber has many E-alkenes, whereas unvulcanized rubber is all Z-alkenes. The sulfur radicals do not add to the alkenes but rather abstract allylic hydrogen atoms. Writing only a small section of rubber:
The new form allylic radical captured one of the sulfur ring presents (S5-S8).
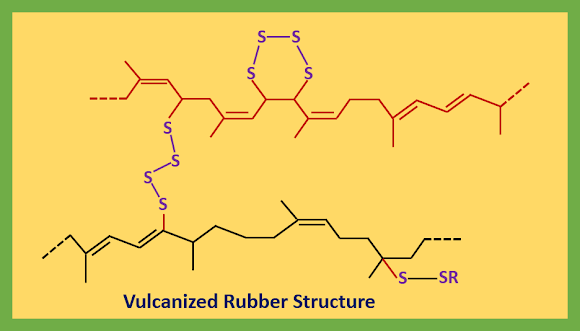
The sulfur radicals can attack another chain to give a cross-link or bite back to give a link within the same chain. Many types of different sulfur links are formed and the next diagram summarizes a part of the vulcanized rubber structure.











No comments:
Post a Comment