Carbonic anhydrase
The enzyme Carbonic anhydrase catalyzes the reaction:
CO2 (aq) + OH- <=====> HCO3-
Without the catalyst, the reaction occurs above PH
= 9, but in presence of the enzyme Carbonic anhydrase the reaction occurs at PH
= 7. At PH
= 7, the hydration of CO2
takes very slowly at room temperature in the absence of any catalyst:
CO2
(aq) + OH- <=====> HCO3- + H+
(pCO2 =1 atm; k= 10-1s-1)
The enzyme Carbonic anhydrase enhances the rate of hydration of CO2 by a factor of about 107
or more. The enzyme Carbonic anhydrase can also catalyze the hydrolysis of esters and aldehydes.
Carbonic anhydrase catalyzes the interconversion of carbon dioxide and carbonates. Carbonic anhydrase has one zinc atom per molecule and it coordinates to three histidine residues (His 94, His 96, His 119) and a water molecule or hydroxide ion, it is represented as L3Zn2+OH-,
where L = imidazole N from histidine. Experimentally, the enzyme Carbonic anhydrase loses activity below PH
=7, it is known that the product of the hydration of CO2
is HCO3-, as would be expected in neutral or basic solution.
Calculation have shown that water bound to the zinc ion can lose a proton readily, but imidazole bound to the zinc ion cannot. There is still an unsettled question about the first ionization from the zinc-bound water molecule. This reaction seems to be much too fast and is dependent on buffer concentration. The role of the buffer is still unknown, but in some fashion, it assists in the reaction. The sequence of reactions usually used to describe the reaction is as follows:
L3Zn2+OH- + CO2
-------> L3Zn2+OH-•CO2 ---(1)
L3Zn2+OH-•CO2
------> L3Zn2+HOCO2- ---(2)
L3Zn2+HOCO2- ------>
L3Zn2+OCOOH- ---(3)
L3Zn2+OCOOH- + H2O --> L3Zn2+(OCOOH-) (H2O)
----(4)
L3Zn2+(OCOOH-) (H2O)
------> L3Zn2+OH2 + HCO3-
-----(5)
L3Zn2+OH2 -------> L3Zn2+OH-
+ H+
(Which may be on a histidine N) ---(6)
The complex formed in (1) is loosely bound, moving to the more tightly bound product of (2). The transition state of reaction (3) may be a bidentate hydrogen carbonate, or there may be a proton transfer between the bound oxygen atom and one of the unbound oxygen atoms. In either case, the result is probably a bound hydrogen carbonate ion that has the OH group at as great a distance from the Zn as possible. Whether reaction (5) has a 5-coordinate Zn with the addition of the water molecule is uncertain; it may just be part of the transition state.
The catalytic action of the zinc-site is initiated by the conversion of a coordinated water molecule to give a Zn-OH bond through the neighbouring histidine and a buffer medium participation. This is followed by a nucleophilic attack on the carbon atom of CO2. The OH group is properly oriented for the attack through a hydrogen bond with a threonine residue in the protein chain (Thr 199). Dehydration of HC18O3-
leaves an 18O on the zinc, suggesting that during the reaction time, the HC18O3- ion must have been directly coordinated to the metal.
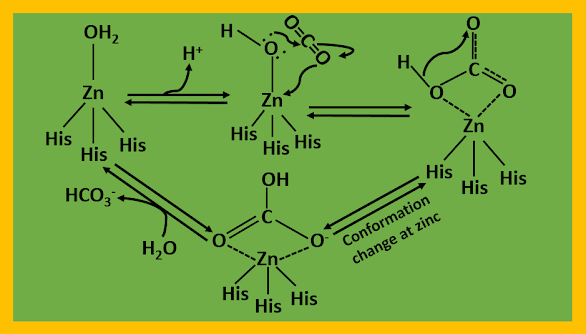 |
| The catalytic path for hydration of carbon dioxide by Carbonic anhydrase |
In the red blood cells, the enzyme Carbonic anhydrase performs the important role of receiving carbon dioxide from tissues such as active muscle and releasing it in the alveoli of the lungs. Each molecule of enzyme Carbonic anhydrase can hydrate about one million of carbon dioxide per second at body temperature (370C). Different closely related forms of the enzyme Carbonic anhydrase are found in mammals, each having the molecular weight 30,000 and a roughly ellipsoidal shape.












No comments:
Post a Comment