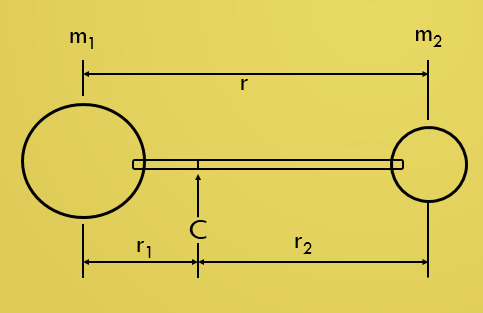
Consider a diatomic molecule in which m1 and m2
are the masses of the two atoms joined by a rigid bar (the bond) whose length
is-----------
r = r1 + r2 ---------------------- (1)
Rotating about an axis passing through its centre of gravity.
The centre of gravity is defined by the equality of the moments about it, that is ----------
m1r1 = m2r2 -------------------- (2)
I = m1r12
+ m2r22
= m2r2r1 + m1r1r2 (From Eq 2) -------(3)
= r1r2 (m1 + m2)
From equation (1) and (2) we get --------
m1r1 =
m2r2 = m2(r-r1)
Therefore,
r1 = (m2r)/(m1+m2) and r2 = (m1r)/( m1+m2) ---- (4)
Replacing (4) in (3) we get,--------------
I = [(m1m2)/(m1+m2)]r2 = µr2 ------- (5)
where, µ = [(m1m2)/(m1+m2)],
and µ is called the reduced mass of the molecule.
By the use of the Schrodinger equation it may be shown that
the rotational energy levels allowed to the rigid diatomic molecule are given
by the expression---
EJ = [h2/8π2I]J(J+1)
joules where J = 0,1,2----- (6)
Where h is Planck’s constant, and I is moment of inertia and
J is rotational quantum number.
We can write the equation (6) as -----
εJ = EJ/hc = [h/8π2Ic]J(J+1)cm-1(J = 0,1,2--) (7)
Where c is the velocity of light expressed in cm/sec.
We can write the equation (7) as---
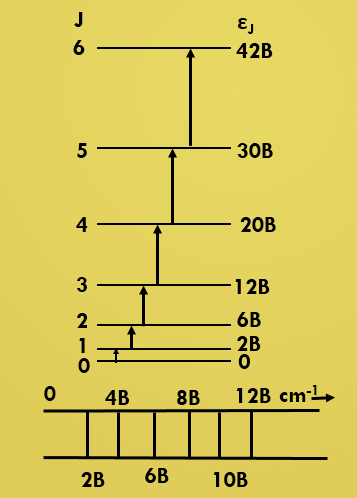
εJ = BJ(J+1) cm-1 (J = 0,1,2,----)
Where B , the rotational constant, is given by -----
B = [h/8π2Ic] cm-1
The rotational transitions for a rigid diatomic molecule are
governed by the selection rule-----
ΔJ = ± 1
That is only those transition are allowed in which the rotational quantum number changes by unity. The + sign refers to absorption and the – sign to emission of radiation.
For a transition taking place from J to J+1, the rotational
frequency is given by-----
ν(J→J+1) =
B(J+1)(J+2)- BJ(J+1)
= B(J2
+3J+2)- B(J2 +J)
= 2B(J+1) cm-1
Thus, ---------------
ν(0→1) =
2B
ν(1→2) =
4B
ν(2→3) =
6B
ν(3→4) =
8B
We see that rotational spectrum of a rigid diatomic molecule
consists of a series of lines at 2B, 4B, 6B, 8B etc.These lines are spaced by an amount of 2B.
Rotational spectrum of a rigid diatomic molecule.











No comments:
Post a Comment