Hemerythrin (Hr)
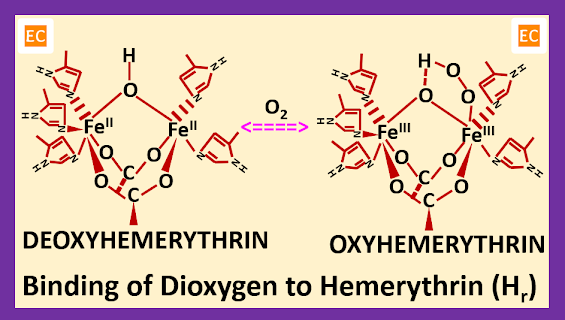
Hemerythrin (Hr) is a nonheme iron containing protein which is a dioxygen-binding pigment found in marine invertebrates, such as sipunculids (peanut marine worms). Hemerythrin (Hr) has some similarities with hemoglobin (Hb) and myoglobin (Mb), and also has some differences with hemoglobin (Hb) and myoglobin (Mb). Several oligomers of hemerythrin (Hr) are known, among them diiron subunit is common. Like both hemoglobin (Hb) and myoglobin (Mb), hemerythrin (Hr) also contains Fe (II) which binds oxygen molecule reversibly, but when hemerythrin (Hr) oxidized to methemerythrin Fe (III), it does not bind dioxygen. There are monomeric, dimeric, trimeric, and tetrameric forms in the tissues with lower molecular weight and octameric form with a molecular weight of about 108,000 which transport dioxygen in the blood. Hemoglobin (Hb) contains four chains, every chain is very similar to the single chain of myoglobin (Mb), and octameric form of hemerythrin (Hr) contains eight subunits very similar in quaternary structure to myohemerythrin. In case of binding of dioxygen there are major differences between hemoglobin (Hb) and hemerythrin (Hr). In hemerythrin (Hr), whether it is in monomeric form or in octameric form each dioxygen binding site contains two Fe (II) atoms, and the reaction takes place via an oxidation-reduction reaction to form Fe (III) and peroxide (O―O)2-. Oxyhemerythrin is diamagnetic in nature with antiferromagnetically coupled Fe (III). In oxyhemerythrin, two Fe (III) atoms are in different environments proved by Mössbauer spectroscopy.
The active site of the reduced deoxy form of hemerythrin (Hr) contains two Fe (II) atoms, separated by roughly 3.25 – 3.5 A0. In the deoxy form of hemerythrin (Hr), the core is asymmetric, one Fe (II) atom have five coordination sites, this Fe (II) atom linked with two histidine side-chain residue, and bridged by one hydroxo group derived from water and aspartate and glutamate, whereas other Fe (II) atom have six coordination sites, this Fe (II) atom linked with three histidine side-chain residue, and bridged by one hydroxo group derived from water and aspartate and glutamate.
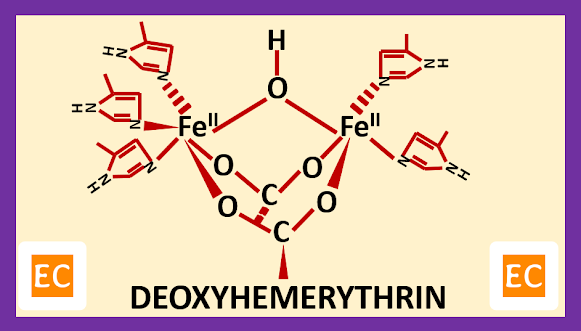 |
| Deoxyhemerythrin |
Peroxide ion [ (O―O)2- ] means dioxygen binds at the vacant site [that Fe (II) atom, which have five coordination sites, so one site is vacant] of the deoxy form of hemerythrin (Hr) and the proton from the hydroxo bridge transferred to the bound O2 (peroxide ion or dioxygen) as two electrons from the Fe24+ core are transferred to O2 (peroxide ion or dioxygen), thus reducing it.
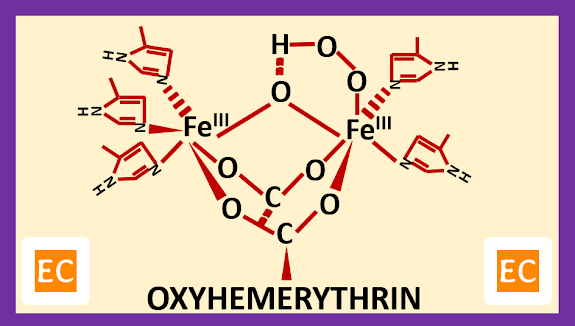 |
| Oxyhemerythrin |
So, in the oxyhemerythrin both Fe (III) atoms have six coordination sites. One Fe (III) atom linked with two histidine side-chain residues, one peroxide ion (O―O)2-, and bridged by one oxygen atom derived from water and aspartate and glutamate, other Fe (III) atom linked with three histidine side-chain residues and bridged by one oxygen atom derived from water and aspartate and glutamate.